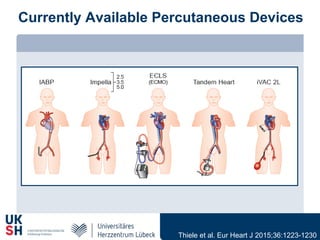
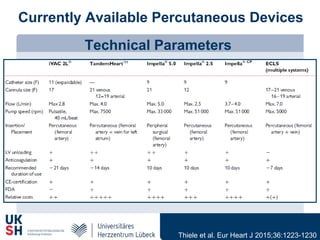
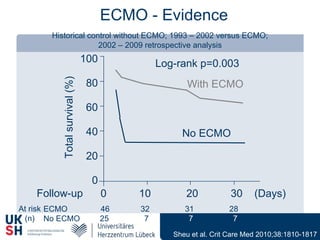
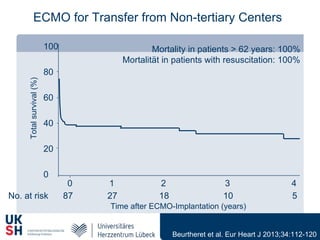
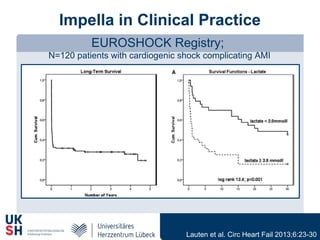
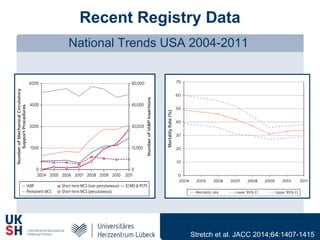
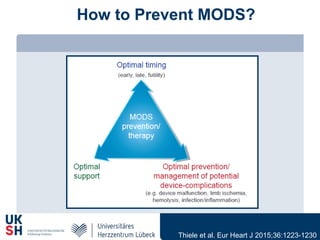
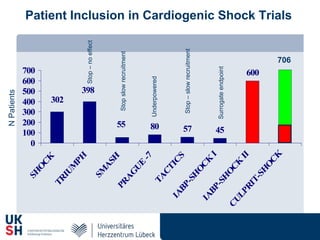
1) Percutaneous circulatory support devices like IABP, Impella and LVADs can provide hemodynamic support in cardiogenic shock, but their effects on mortality are unclear from randomized trials.
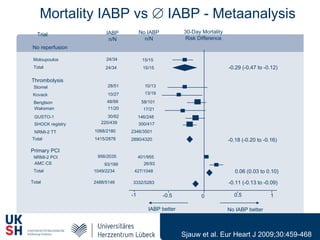
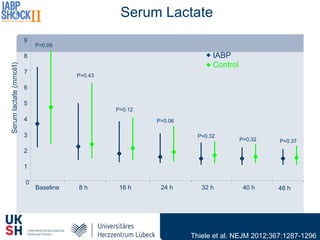
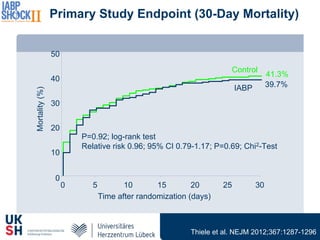
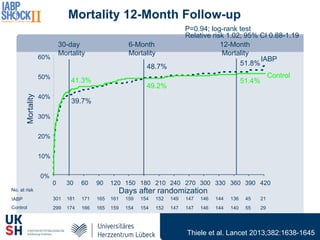
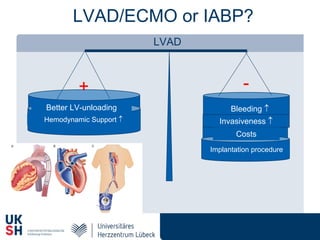
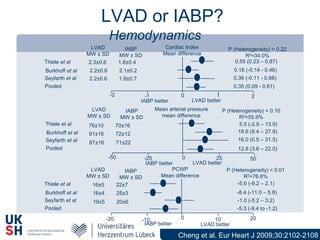
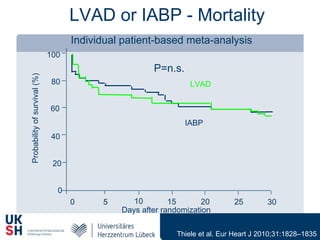
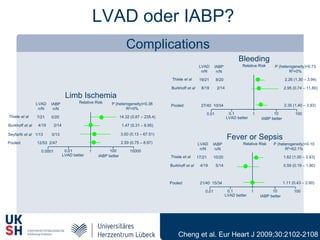
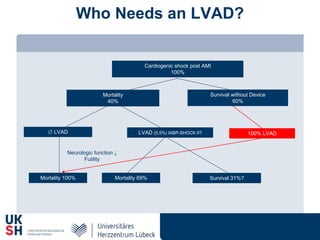
2) While LVADs provide better hemodynamic support than IABP, they also have higher risks of complications like bleeding and limb ischemia. Trials comparing IABP to medical management alone found no significant difference in mortality.
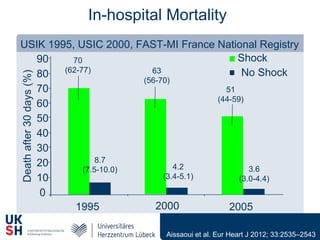
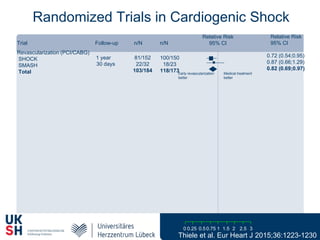
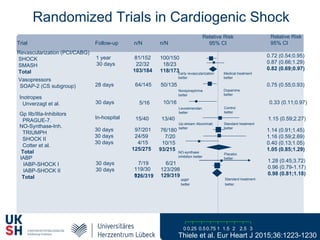
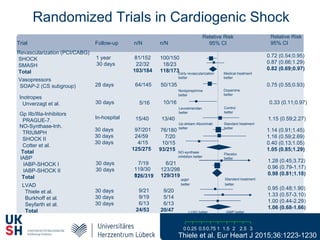
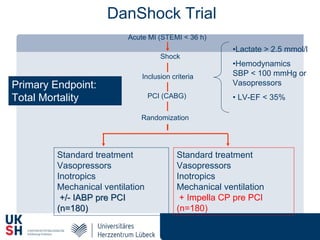
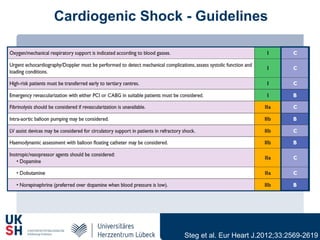
3) Revascularization through PCI or CABG within 36 hours appears to reduce mortality compared to medical stabilization alone for cardiogenic shock patients.