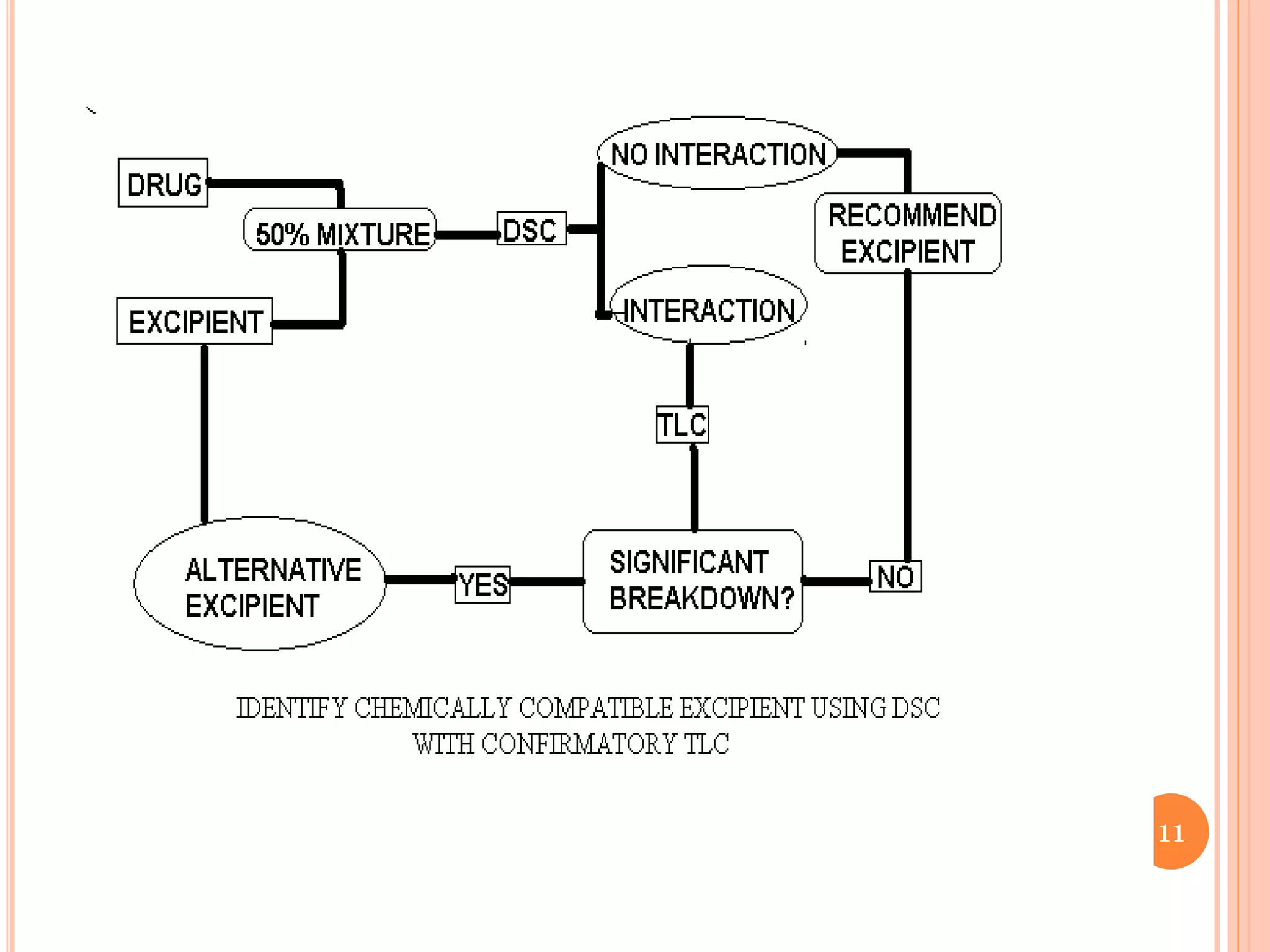
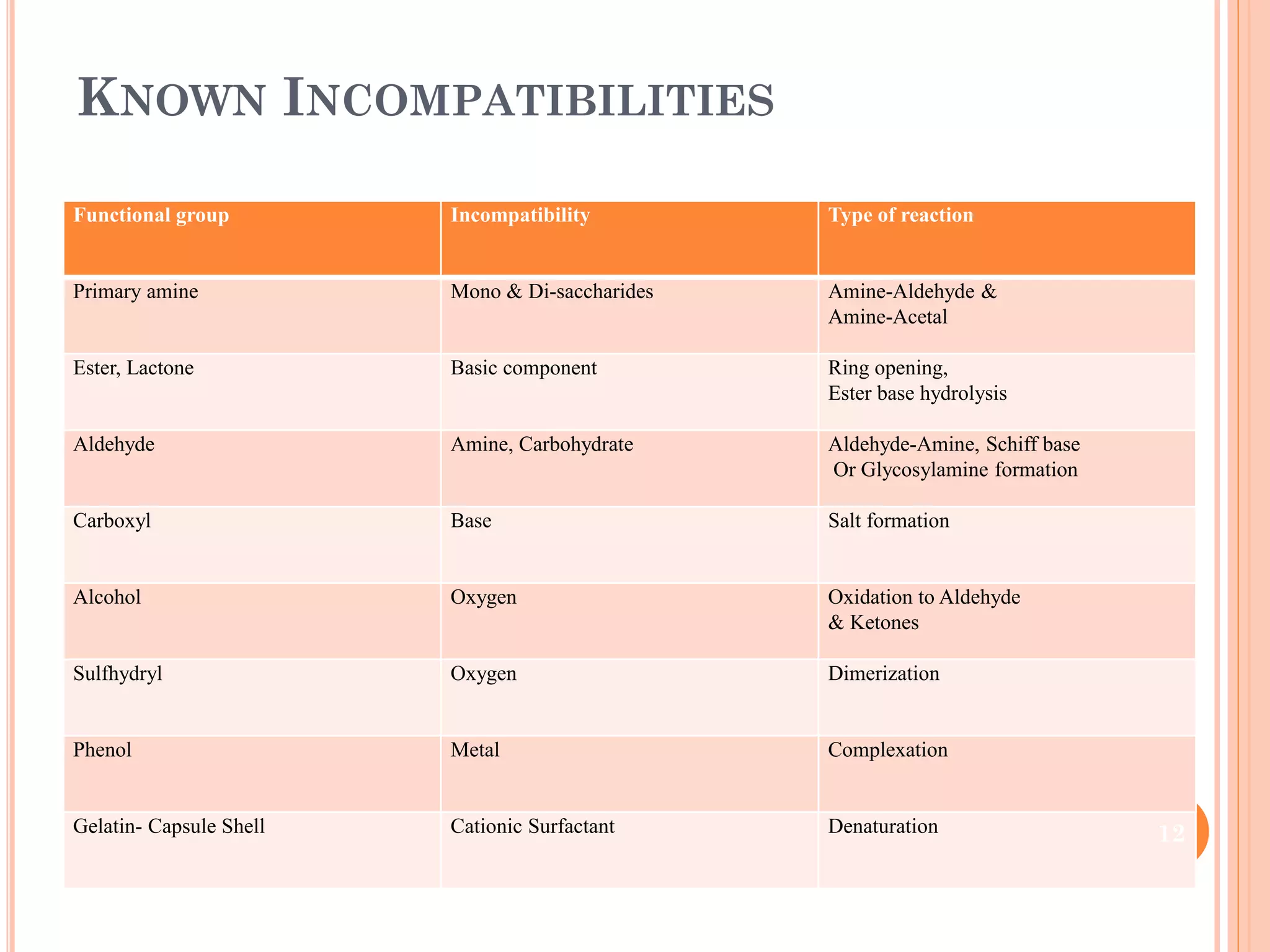
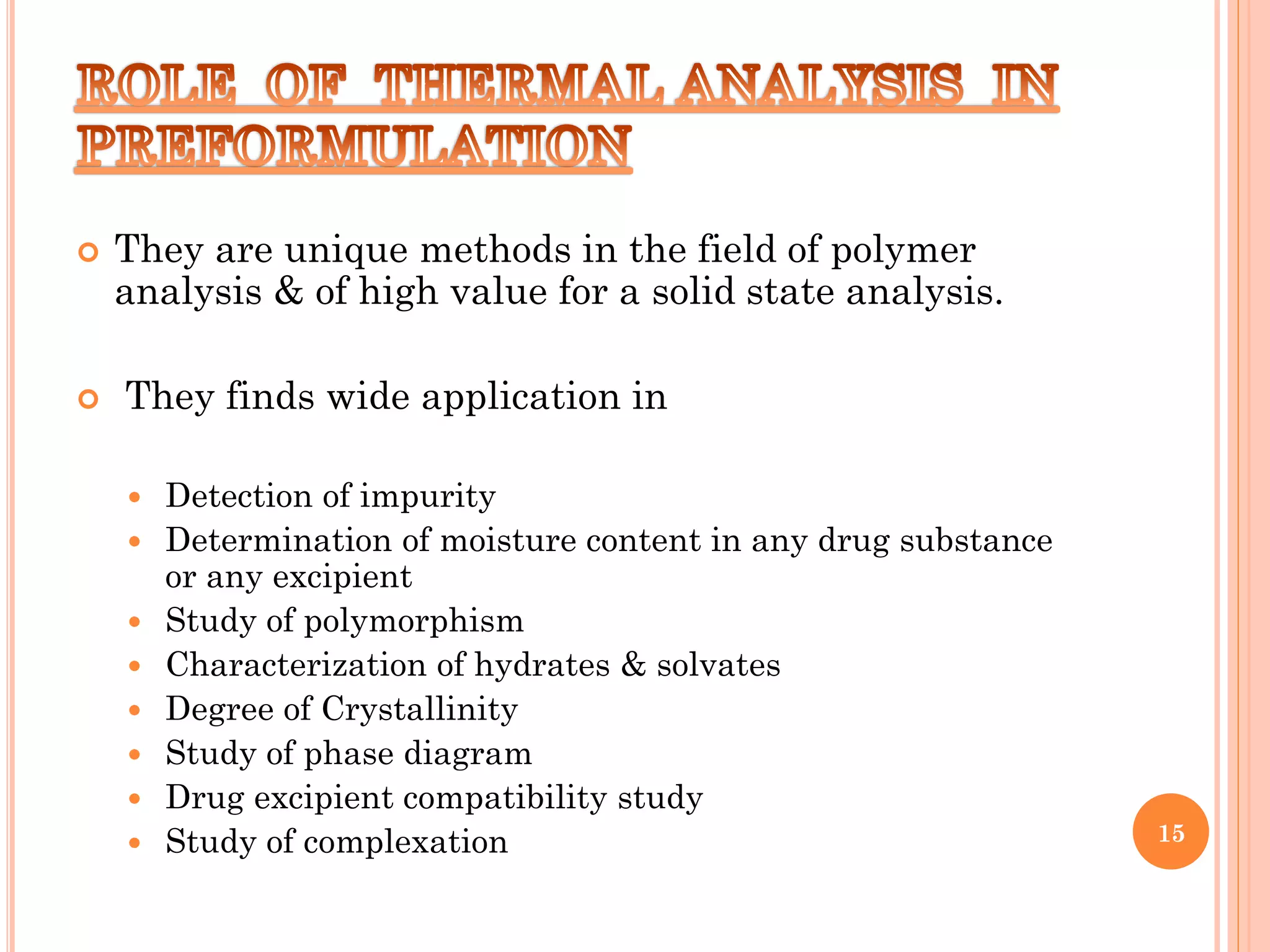
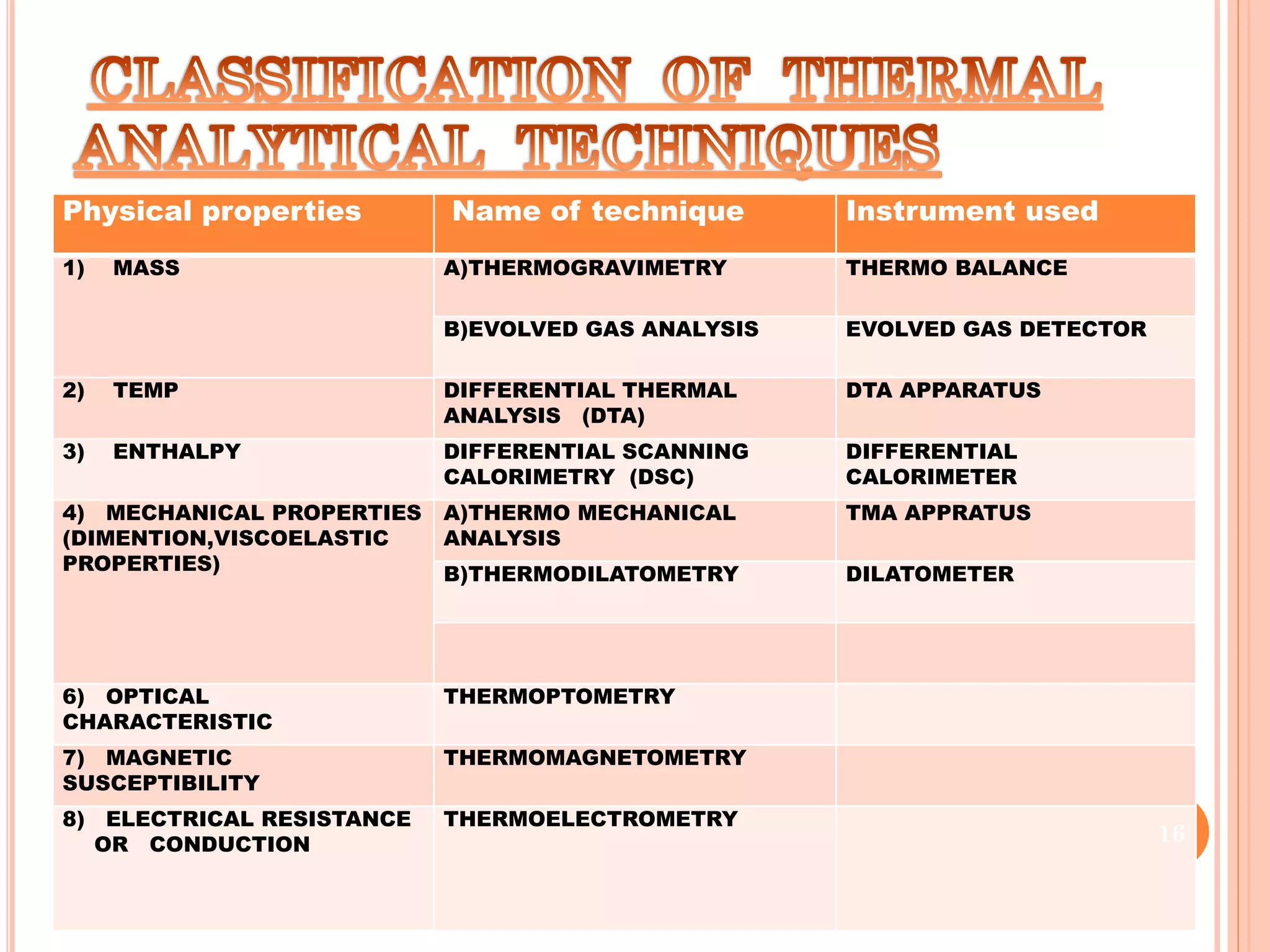
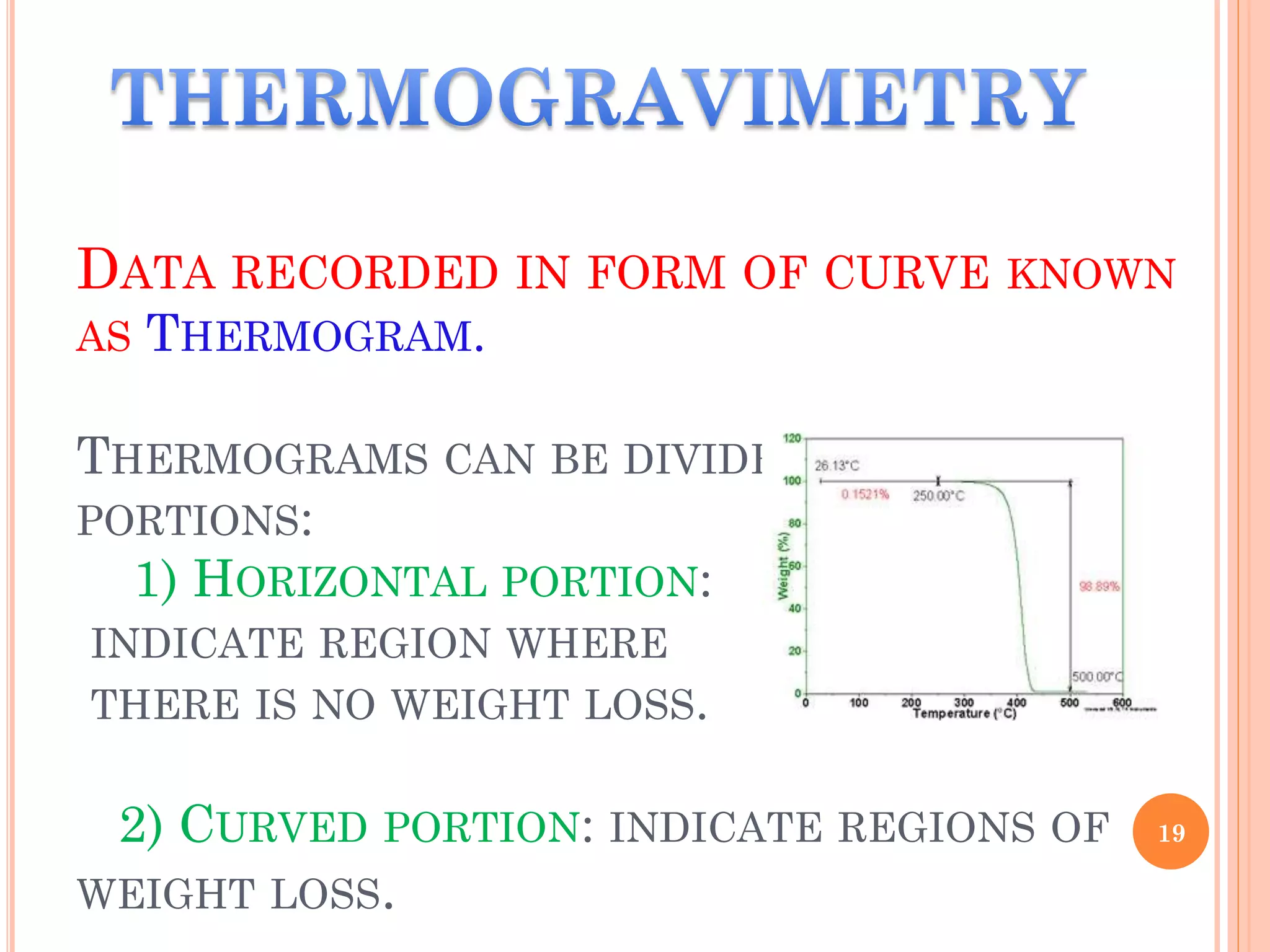
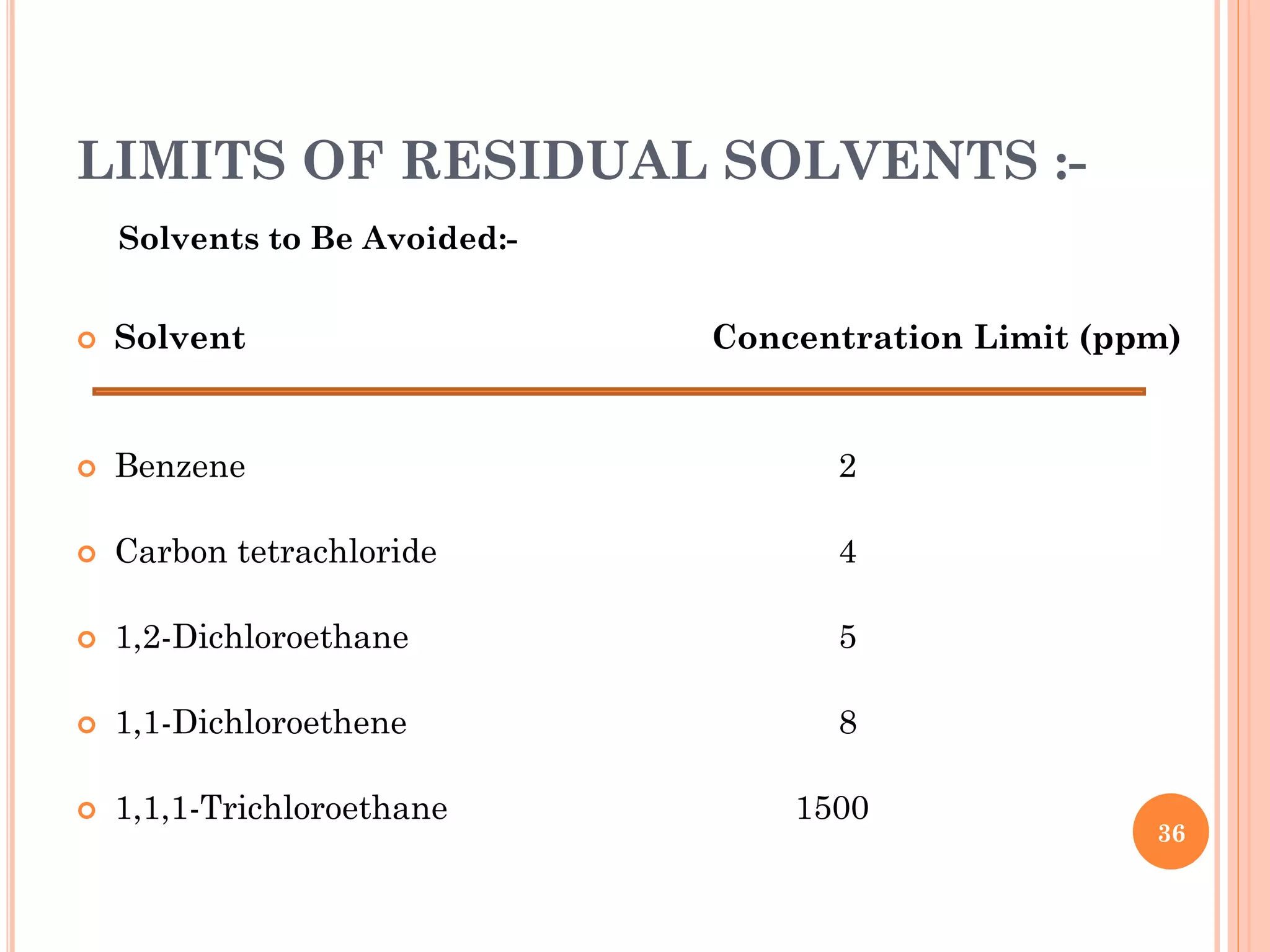
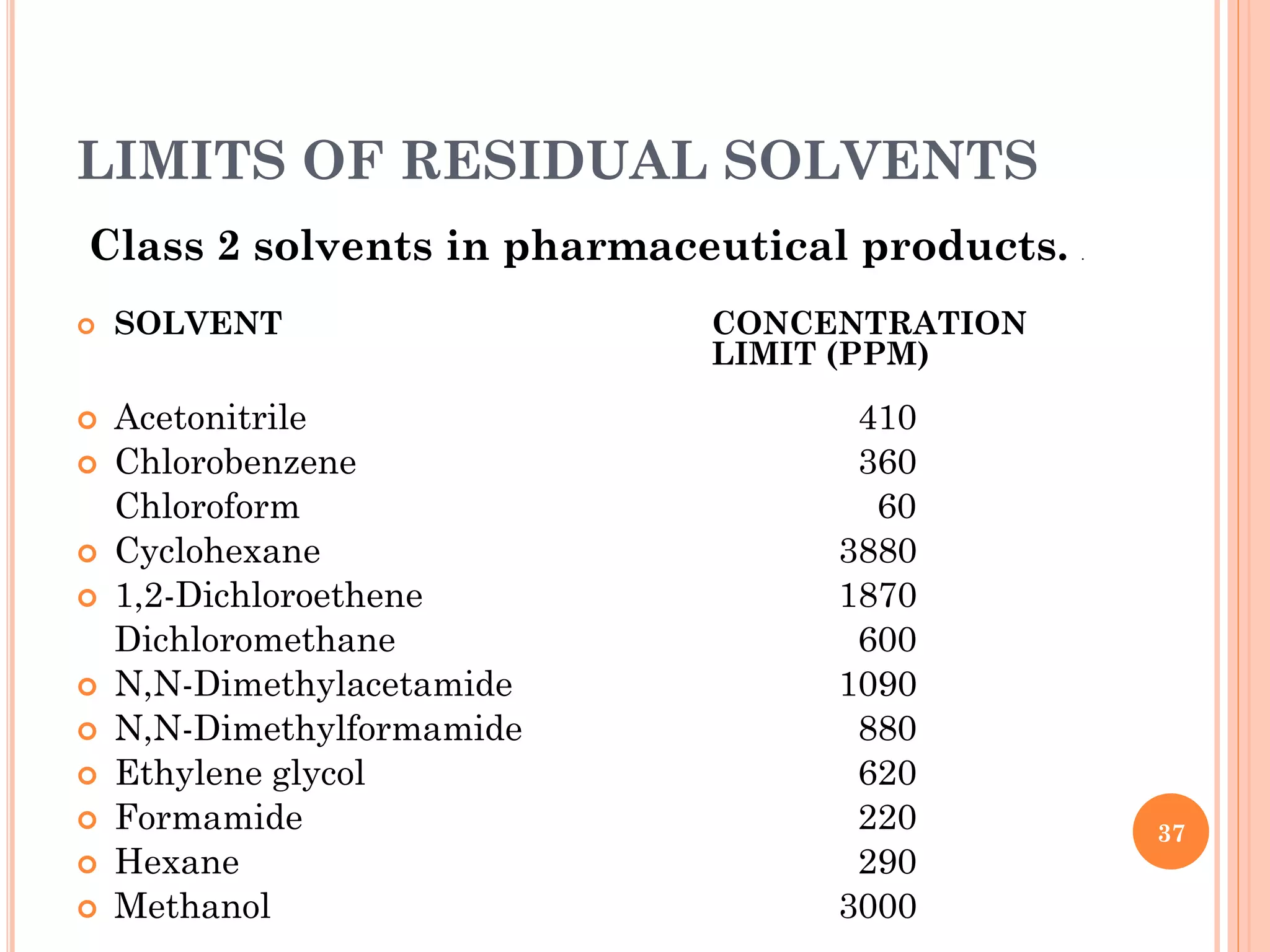
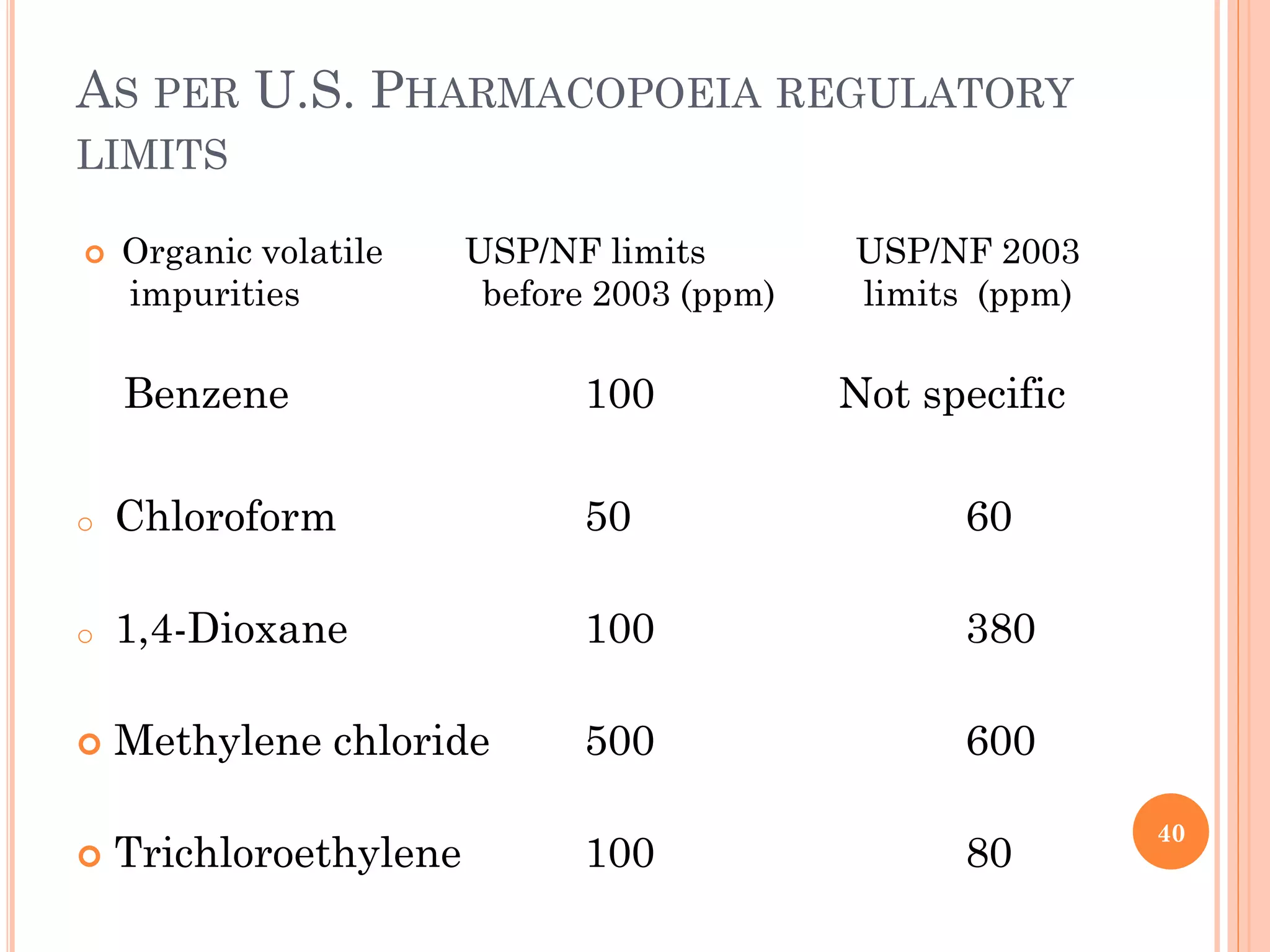
This document discusses preformulation and various analytical techniques used for drug-excipient compatibility studies. It describes types of incompatibilities like physical and chemical incompatibilities. Techniques discussed include thermal analysis methods like thermogravimetry (TG), differential thermal analysis (DTA), and differential scanning calorimetry (DSC). Other techniques mentioned are X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). The document also covers limitations of thermal analysis and applications of these techniques in preformulation studies like characterization of hydrates/solvates, detection of impurities, and compatibility studies.