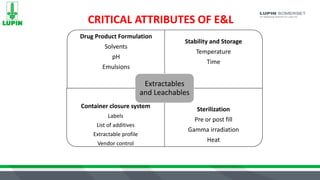
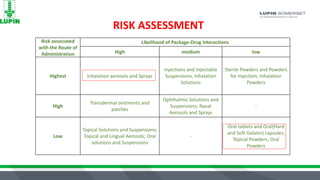
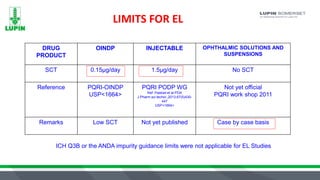
The document discusses extractables and leachables (E&L) in drug packaging, emphasizing regulatory perspectives, analytical methods, and best practices for safety assessments. It outlines the responsibilities of Lupin Somerset's analytical research and development team in investigating these compounds and the implications for product safety and regulatory compliance. Key elements include risk assessment, controlled extraction studies, and the importance of establishing correlations between leachables and extractables to meet FDA requirements.