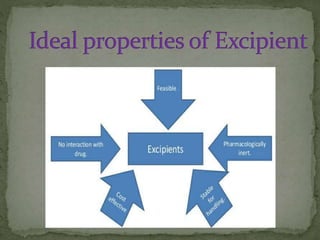
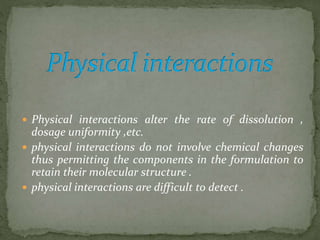
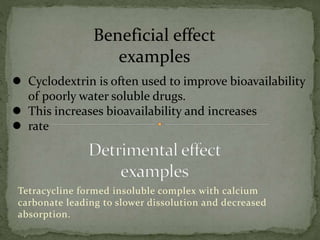
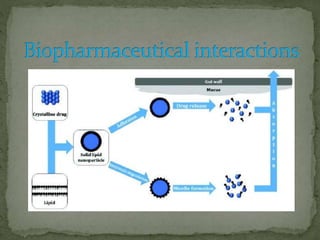
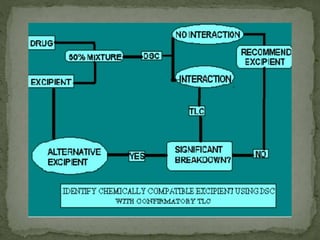
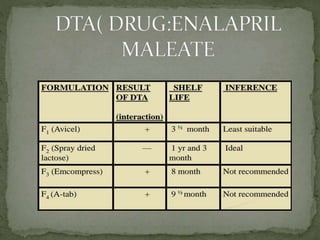
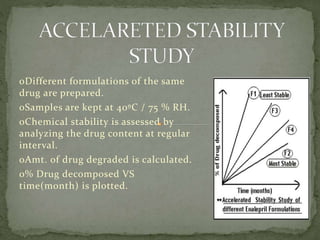
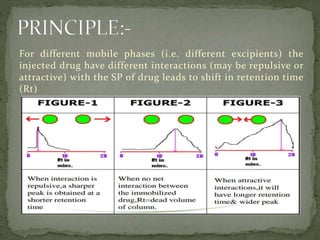
This document discusses excipients and their role in drug formulations. It notes that excipients are ingredients other than the active pharmaceutical ingredient that are used to formulate dosage forms. Excipients can act as protective agents, bulking agents, and can improve drug bioavailability. The document then lists common types of excipients and potential interactions between drugs and excipients, such as physical, chemical, biopharmaceutical, and excipient-excipient interactions. It describes several analytical techniques used to detect drug-excipient interactions, including DSC, accelerated stability studies, FT-IR, DRS, chromatography methods, and others.