The document discusses guidelines for forced degradation studies to develop stability-indicating methods. Key points include:
1) Forced degradation studies subject drug substances, products, and placebos to stress conditions like heat, humidity, acids, bases, oxidation, and light to understand degradation pathways and develop suitable methods.
2) Common stress tests include thermal, hydrolytic, oxidative, and photolytic degradation. Conditions are specified for each test.
3) Potential degradation products are identified and the method is evaluated based on peak purity, mass balance, and other criteria.
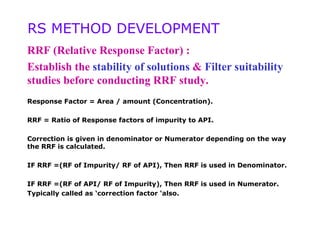
4) Guidelines are provided for establishing relative response factors, quantitation methods, and system suitability parameters based on forced degradation results.