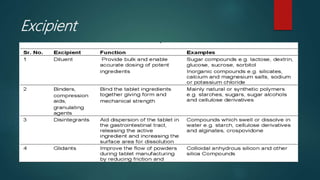
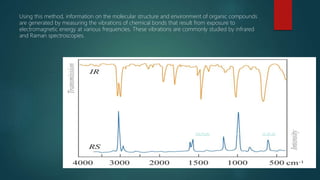
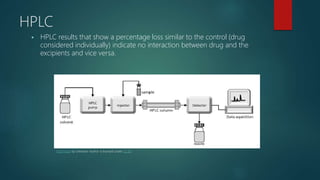
The document discusses drug-excipient compatibility studies, which are important to understand interactions between active pharmaceutical ingredients and excipients. There are three main types of incompatibility - physical, chemical, and therapeutic. Compatibility studies help identify incompatible excipients, ensure excipients do not impact drug stability, and can help stabilize unstable drugs. Methods to study compatibility include thermal techniques like DSC, spectroscopic techniques, microscopy, and chromatography. The goal is to avoid issues with drug stability and efficacy during storage and use.