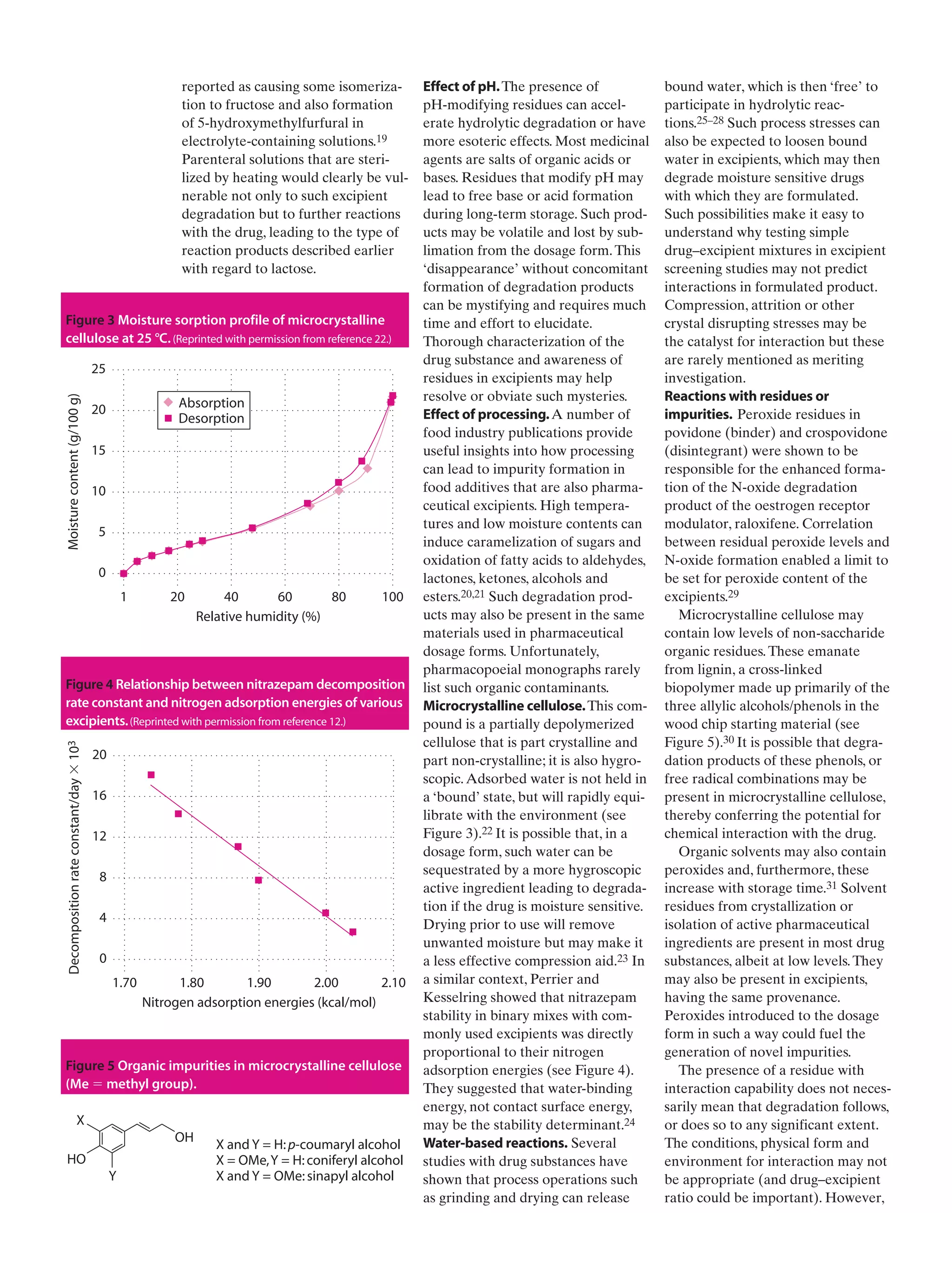
This document discusses how excipients, which are included in drug formulations to aid manufacture and administration, can potentially interact with active drug compounds. While excipients are generally considered inert, they may initiate or participate in chemical or physical interactions that compromise drug quality or effectiveness. Excipients may contain impurities or form degradation products that can cause drug decomposition. Common interaction mechanisms explored include charge interactions, hydrogen bonding, reactions involving functional groups like lactose and amine groups, and physical adsorption effects. The document stresses the importance of understanding excipient properties and potential for interactions to avoid undesirable outcomes in drug formulations.