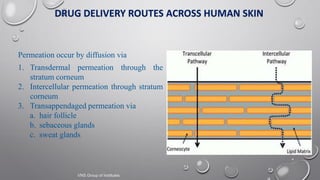
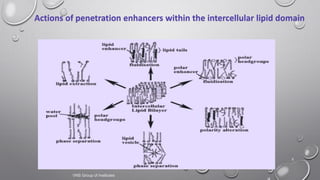
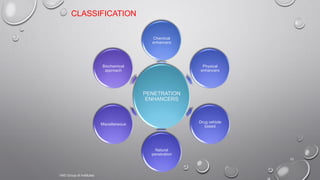
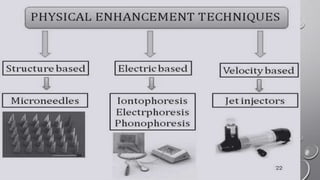
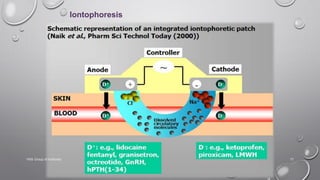
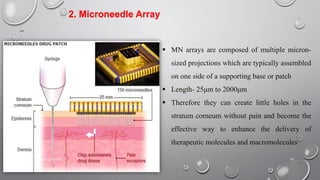
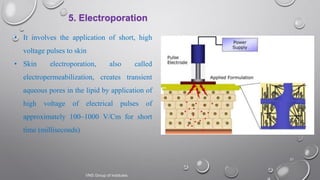
The document discusses penetration enhancers, which are substances that facilitate the absorption of drugs through the skin. It covers their properties, uses, merits, and demerits, as well as various methods of enhancing drug delivery like iontophoresis, microneedle arrays, and sonophoresis. Additionally, it highlights the mechanisms of action and classification of these enhancers, alongside references for further reading.