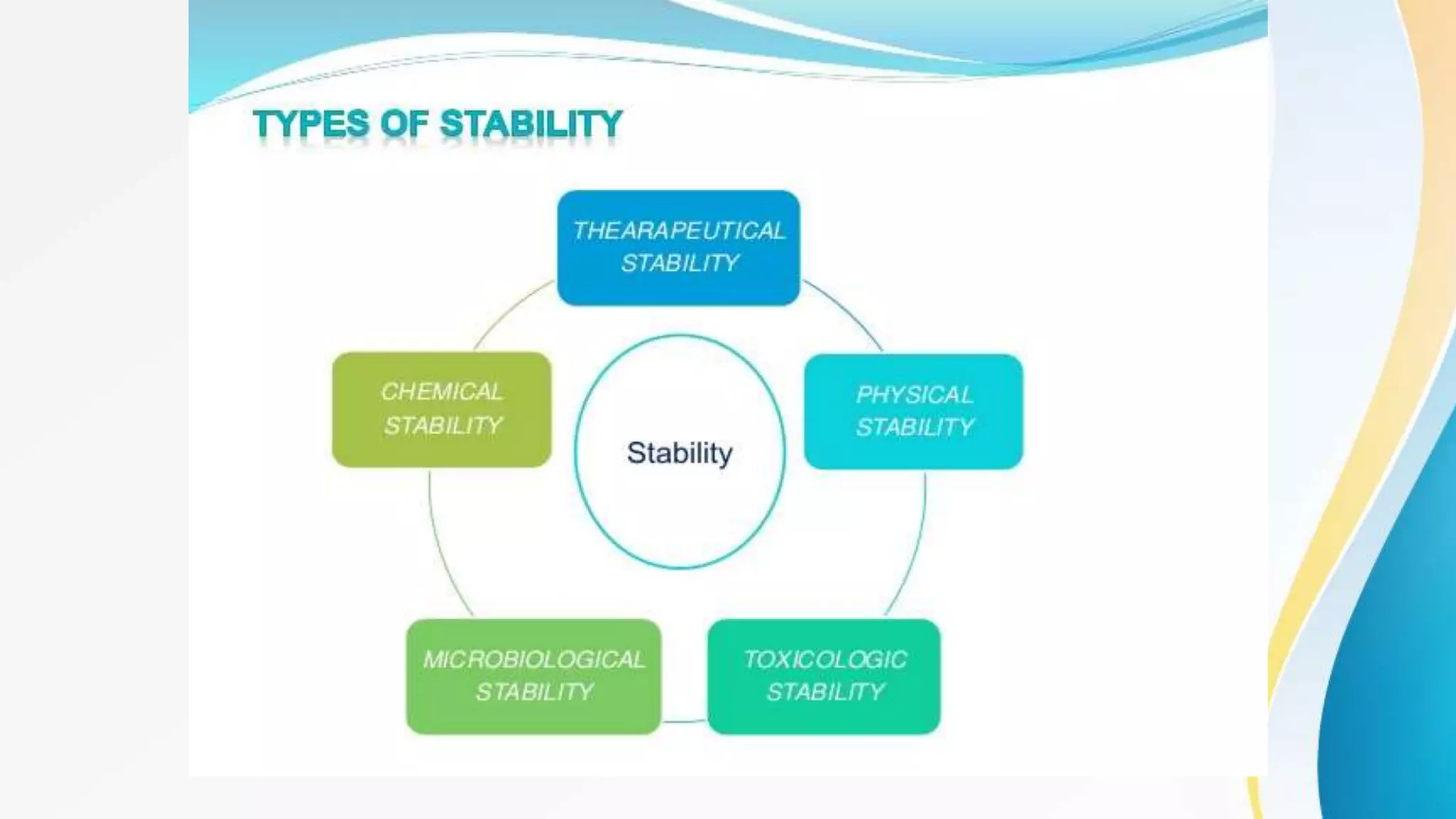
This presentation provides an overview of pharmaceutical stability testing. It discusses the different types of stability studies including formal stability studies, stress stability studies, and abbreviated stability studies. Key factors that influence drug stability are also explained such as temperature, humidity, pH, and light exposure. Common degradation pathways for drugs like hydrolysis, oxidation, and photodegradation are summarized. The presentation emphasizes the importance of stability testing for determining a drug's shelf life and appropriate storage conditions. It provides examples of stability issues for several drugs.

















![ZERO ORDER REACTION
• The rate of reaction doesn't depend on concentration of
reactant(s).
• The rate expression for chemical reaction
A B
Rate of reaction = -d[C]/dt = k
where , k = rate constant
[C]= decreasing conc. of reactant](https://image.slidesharecdn.com/stability-190216122106/75/Product-Stability-Studies-Stability-Testing-25-2048.jpg)
![FIRST ORDER REACTION
• Rate of reaction depends on concentration of any one
reactant
• the rate expression for chemical reaction
A B
rate of reaction = -d[C]/dt = kC](https://image.slidesharecdn.com/stability-190216122106/75/Product-Stability-Studies-Stability-Testing-26-2048.jpg)