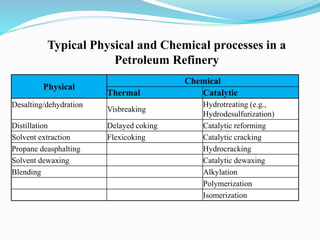
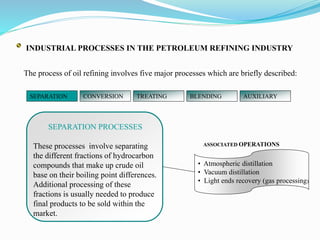
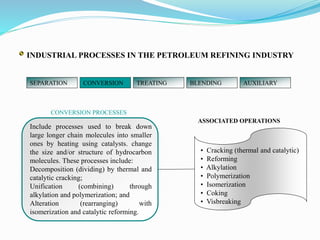
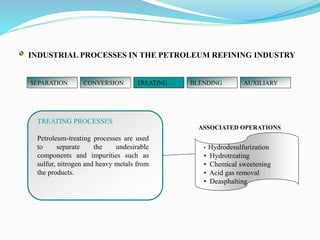
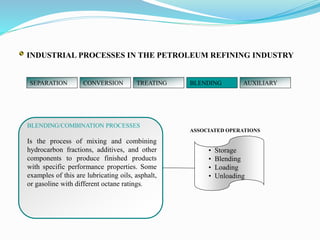
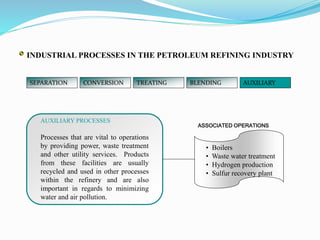
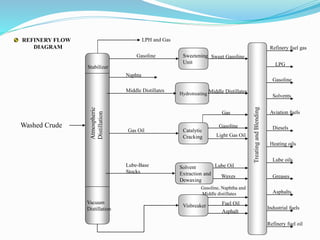
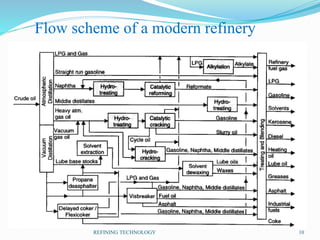
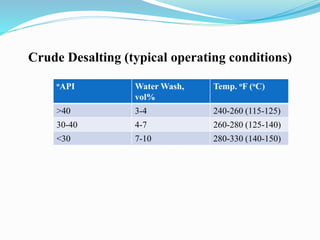
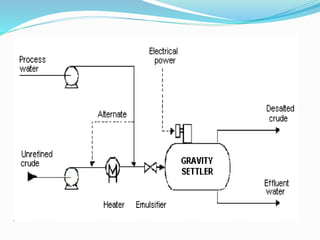
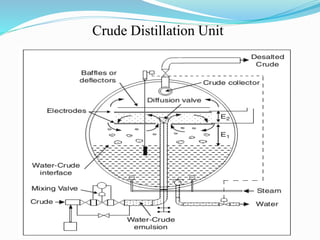
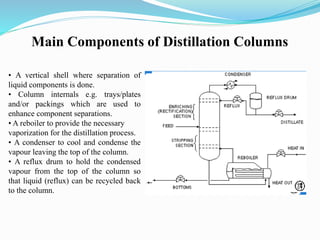
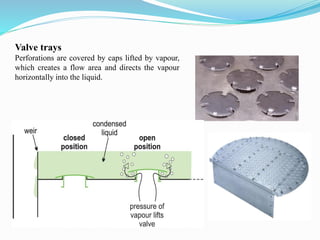
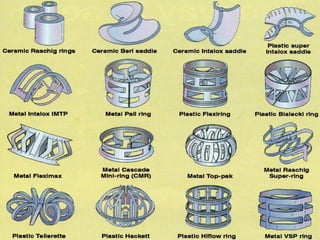
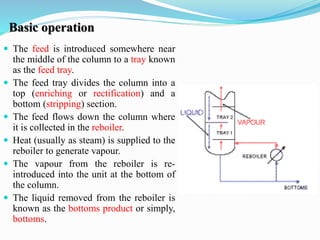
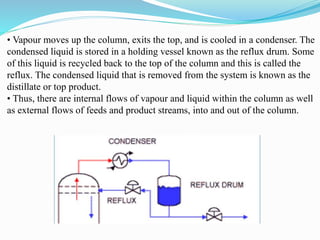
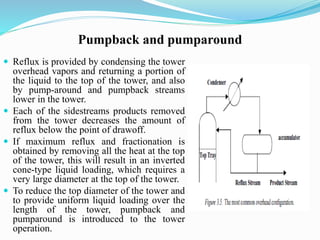
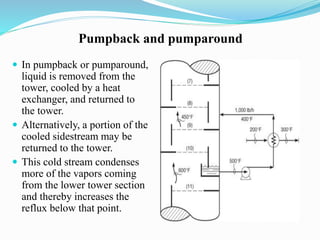
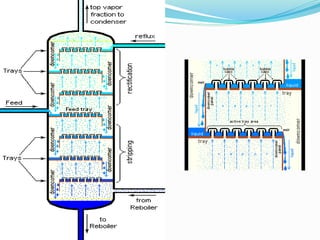
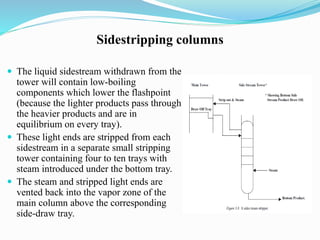
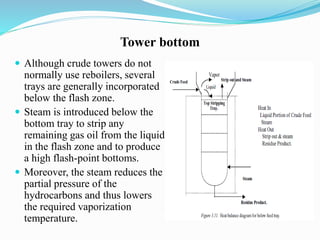
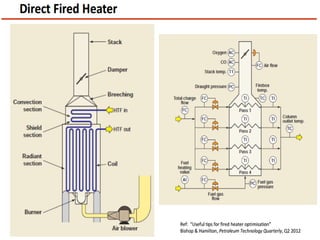
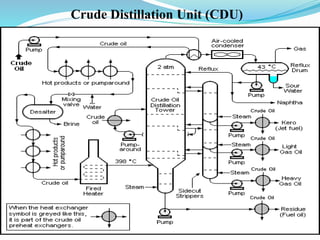
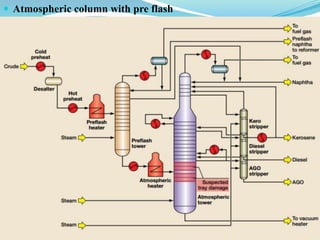
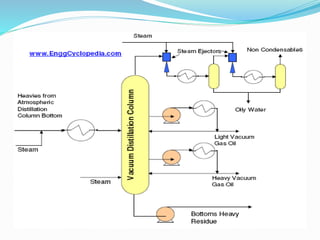
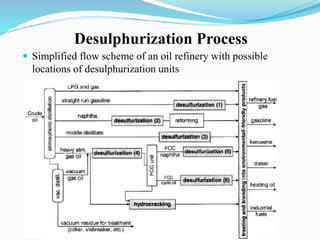
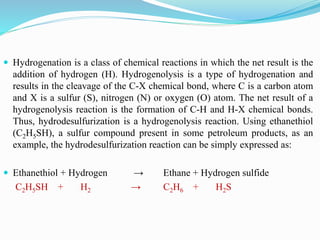
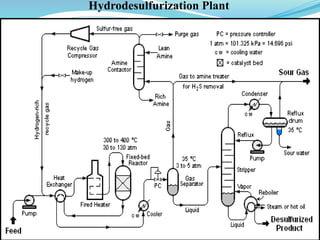
After crude oil is desalted and dehydrated, it is separated into fractions through distillation. However, the distilled fractions cannot be used directly and require further processing due to differences between crude oil properties and market needs. The complexity of refining processes is also due to environmental regulations that require cleaner products. Distillation involves heating crude oil to separate it based on boiling points, but the distilled fractions need additional conversion processes before they can be used or sold.