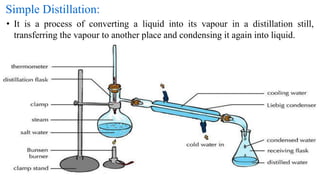
This document discusses various distillation techniques used in pharmaceutical applications. It defines distillation as the separation of components in a liquid mixture through vaporization and condensation. The main types discussed are simple distillation, fractional distillation, steam distillation, and vacuum distillation. Simple distillation involves heating a liquid to its boiling point to produce vapor which is then condensed. Fractional distillation uses a fractionating column to separate close-boiling mixtures. Steam distillation uses steam to distill thermally sensitive compounds. Vacuum distillation reduces pressure to lower the boiling point of compounds. Purified water and water for injection are prepared through distillation to remove impurities, microorganisms, and pyrogens.