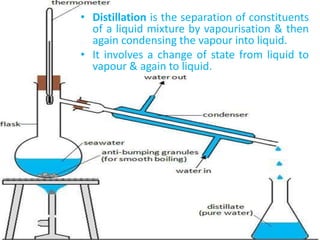
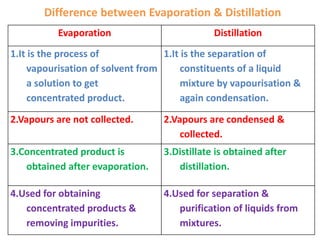
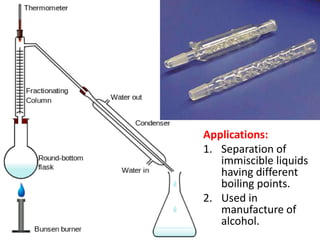
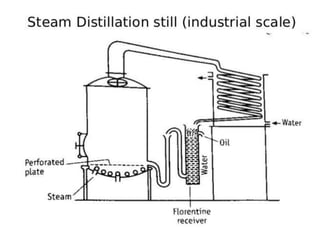
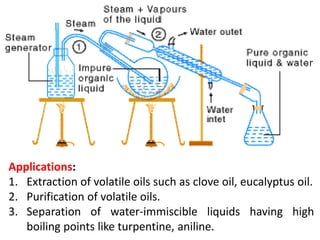
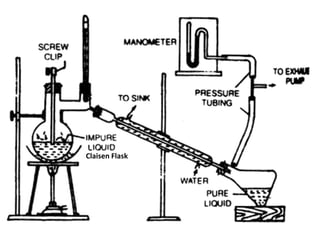
Distillation is a separation process that involves boiling a liquid mixture and condensing the vapor to obtain purified fractions. There are several types of distillation: simple distillation separates compounds based on differences in boiling points; fractional distillation is used for very similar compounds through repeated distillation in a fractionating column; steam distillation utilizes steam to distill compounds that have high boiling points; and distillation under reduced pressure allows heat-sensitive compounds to be distilled at lower temperatures. Distillation has many applications in areas like extracting essential oils, purifying organic compounds, and separating immiscible liquids.