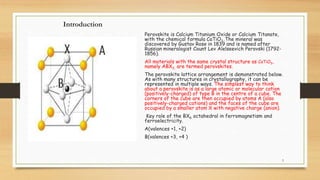
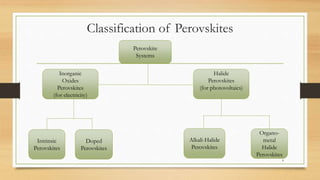
The document discusses perovskites, materials characterized by a specific crystal structure and their diverse applications in electronics and photovoltaics. It covers the structure-property correlations, processing techniques, and the various types of perovskites, as well as their optical and electrical properties. Additionally, it addresses current challenges, such as toxicity and stability issues, faced in the use of perovskites in practical applications.