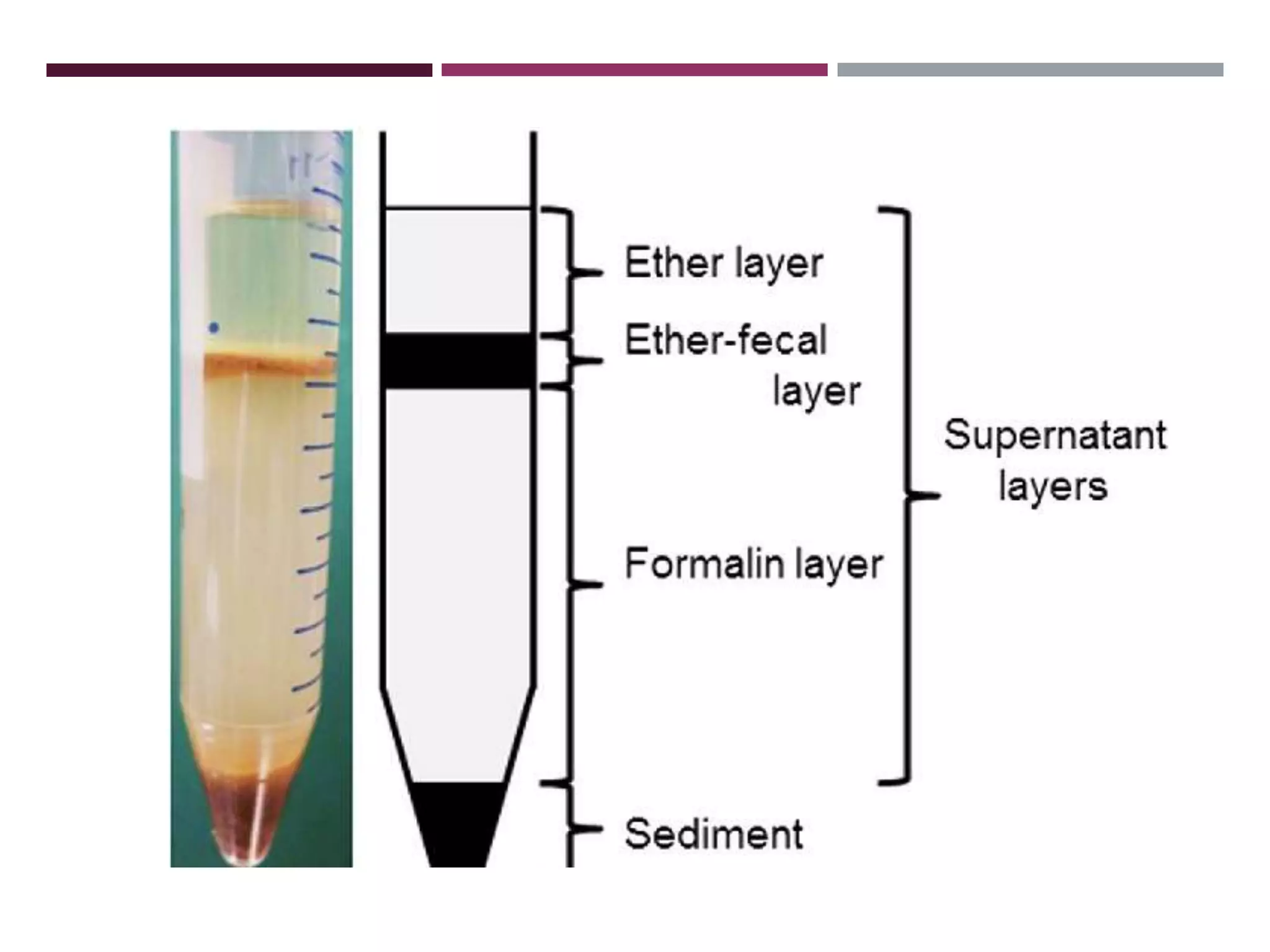
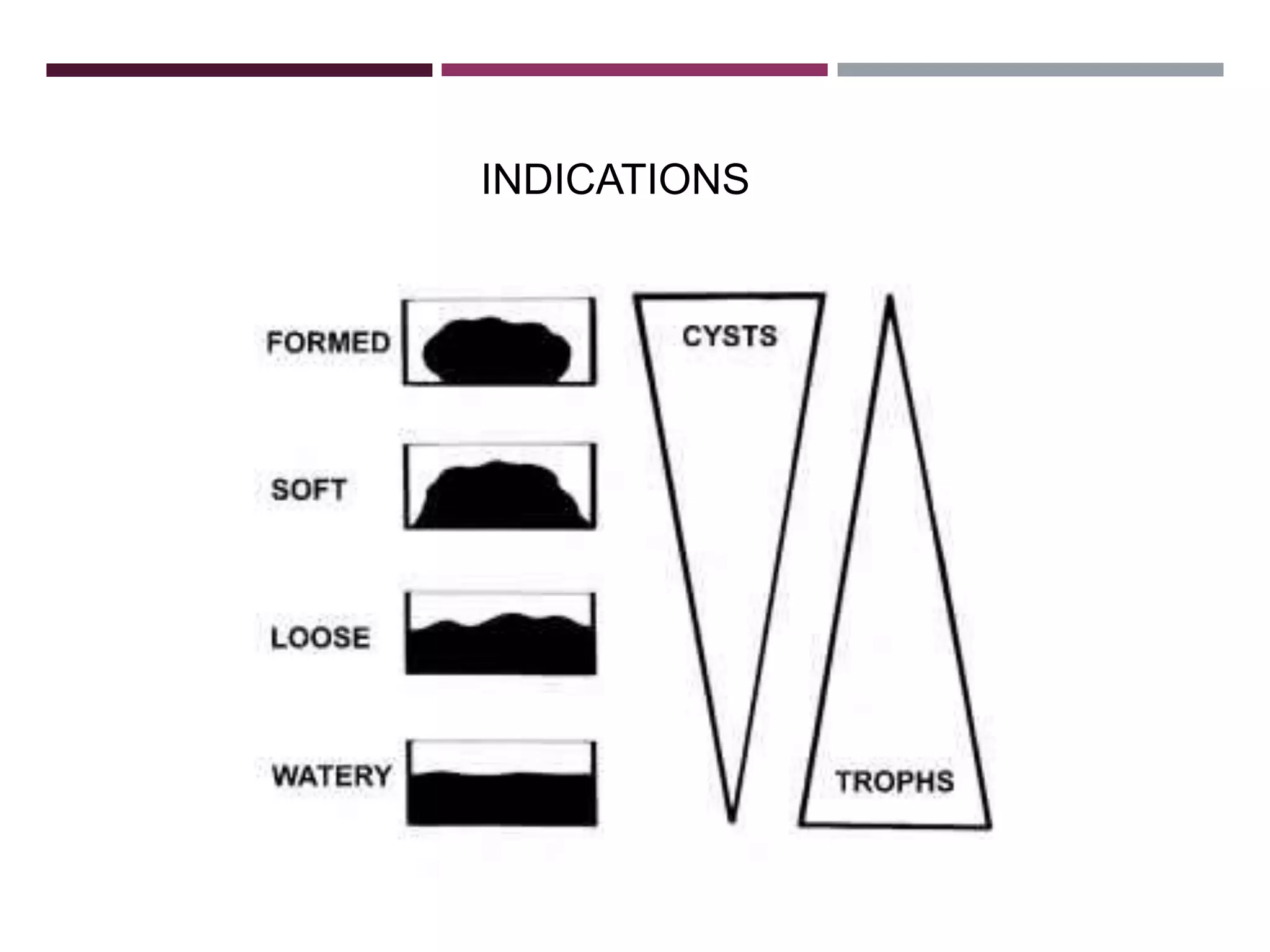
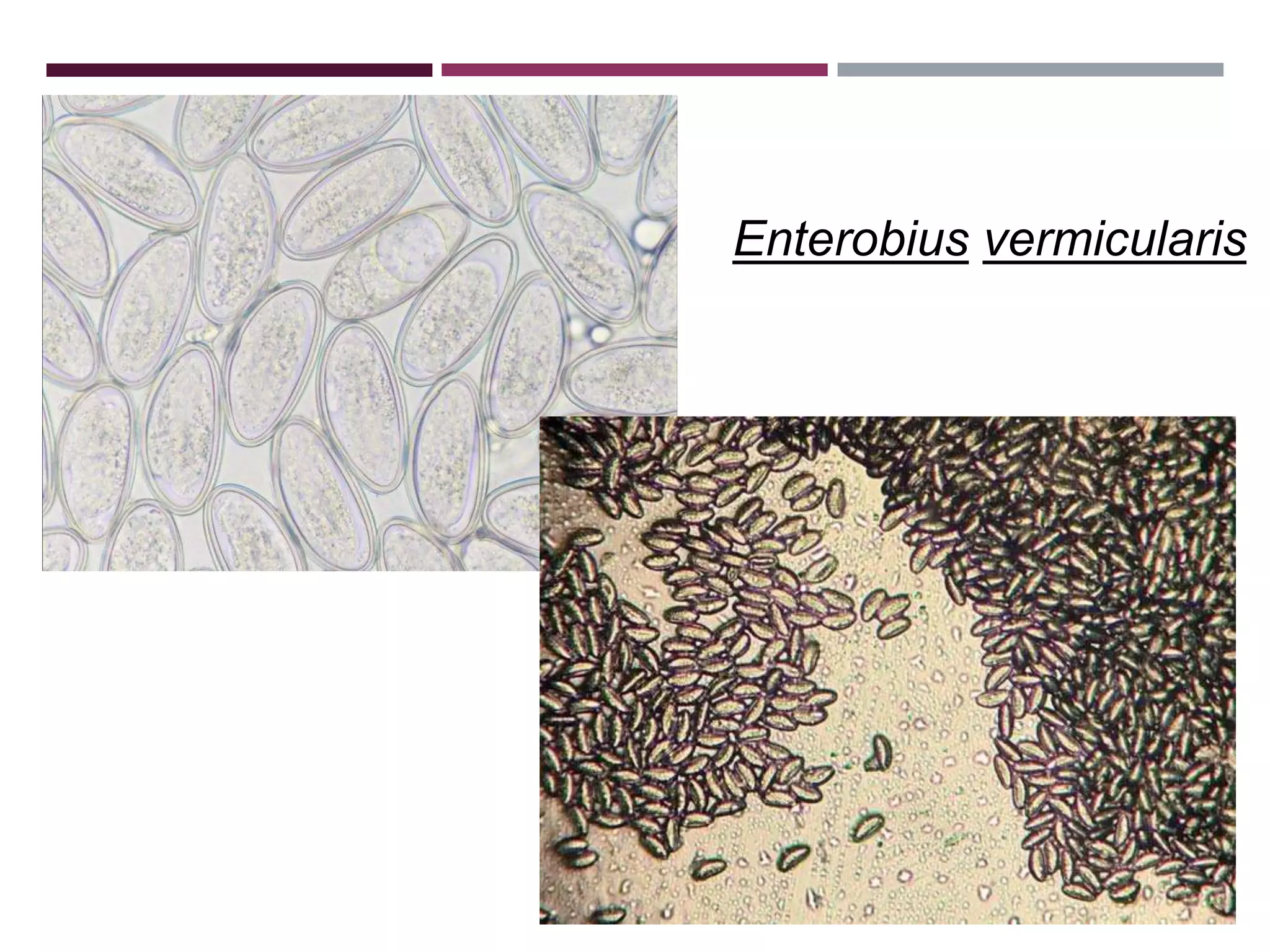
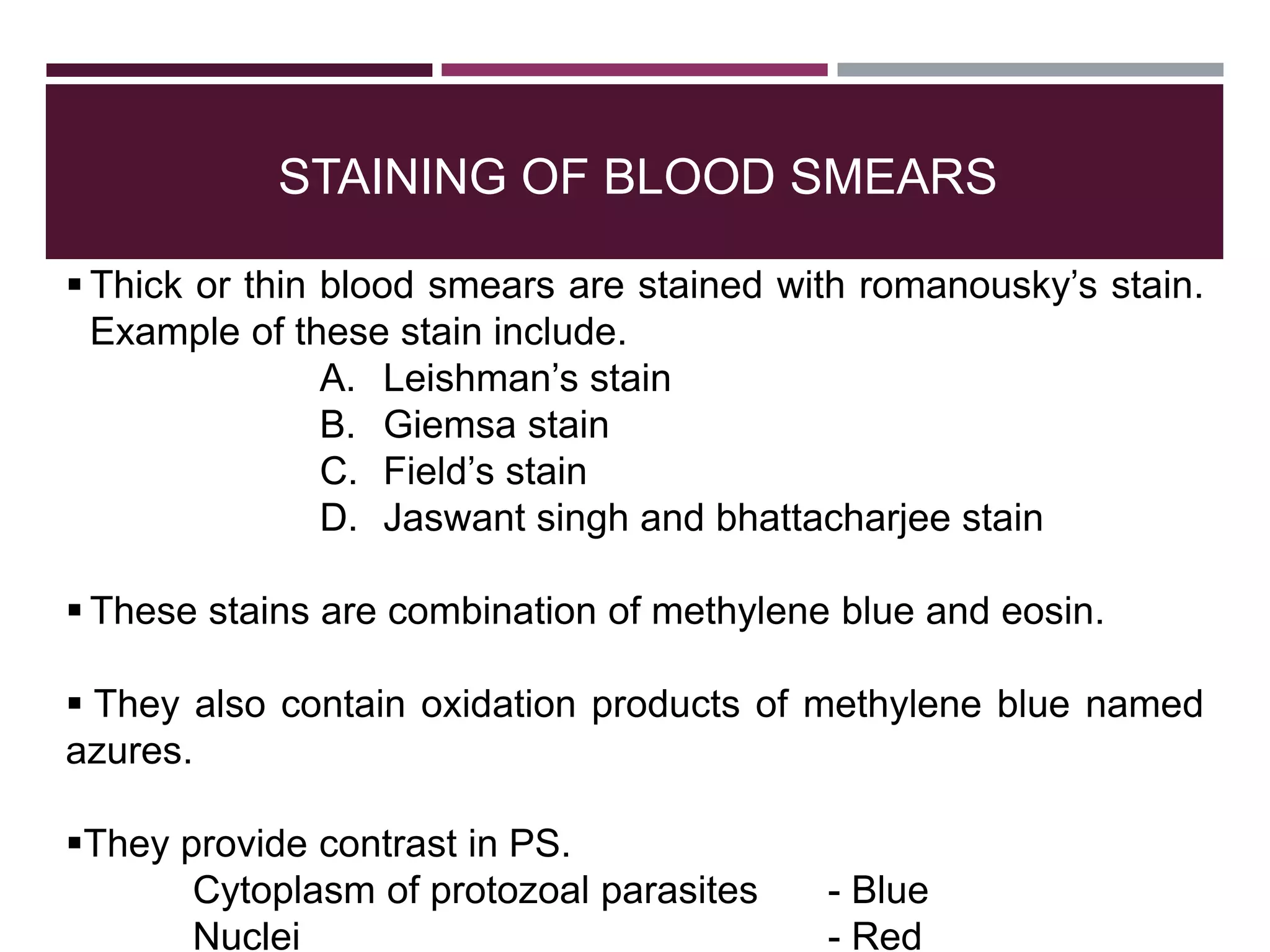
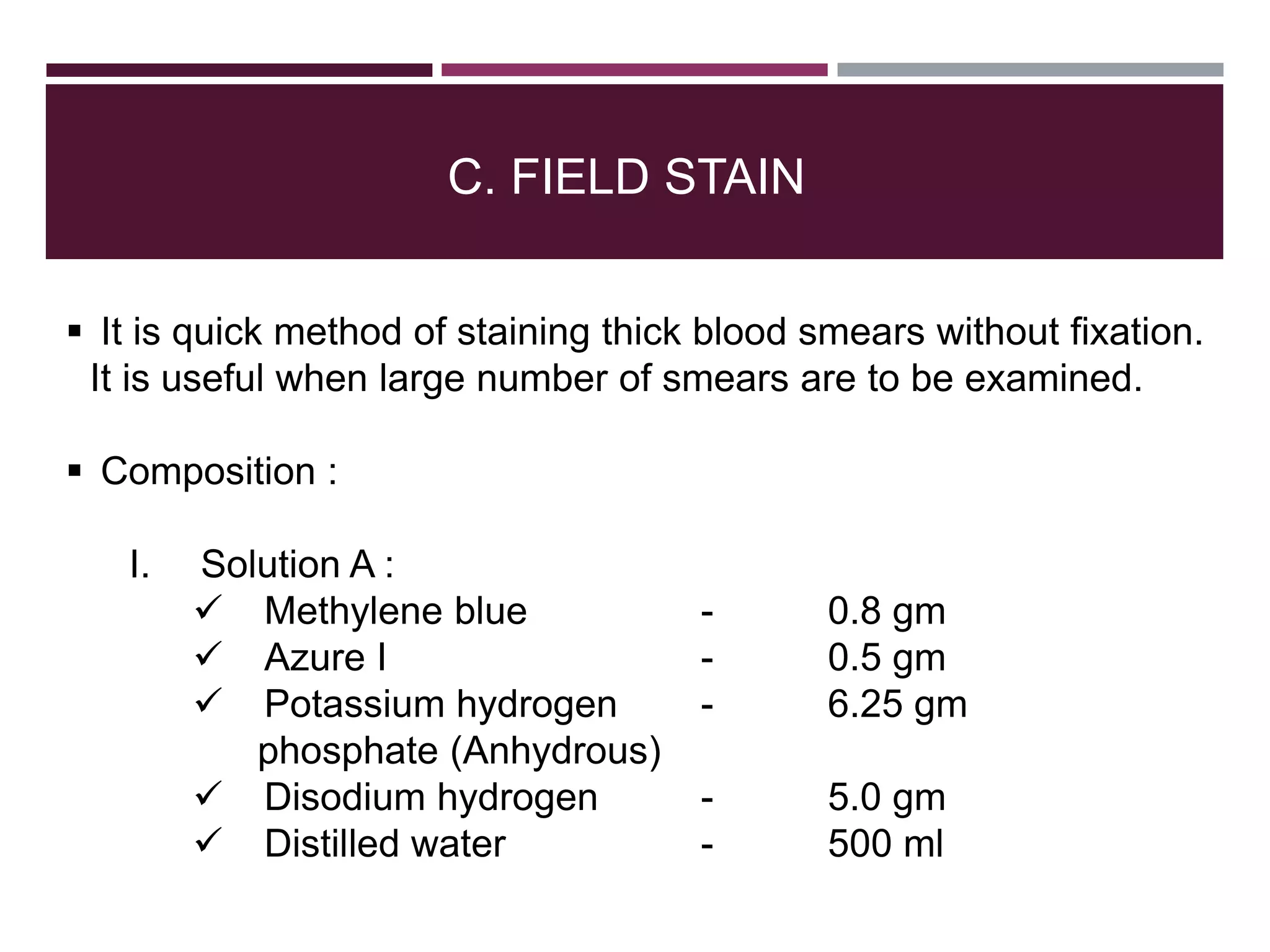
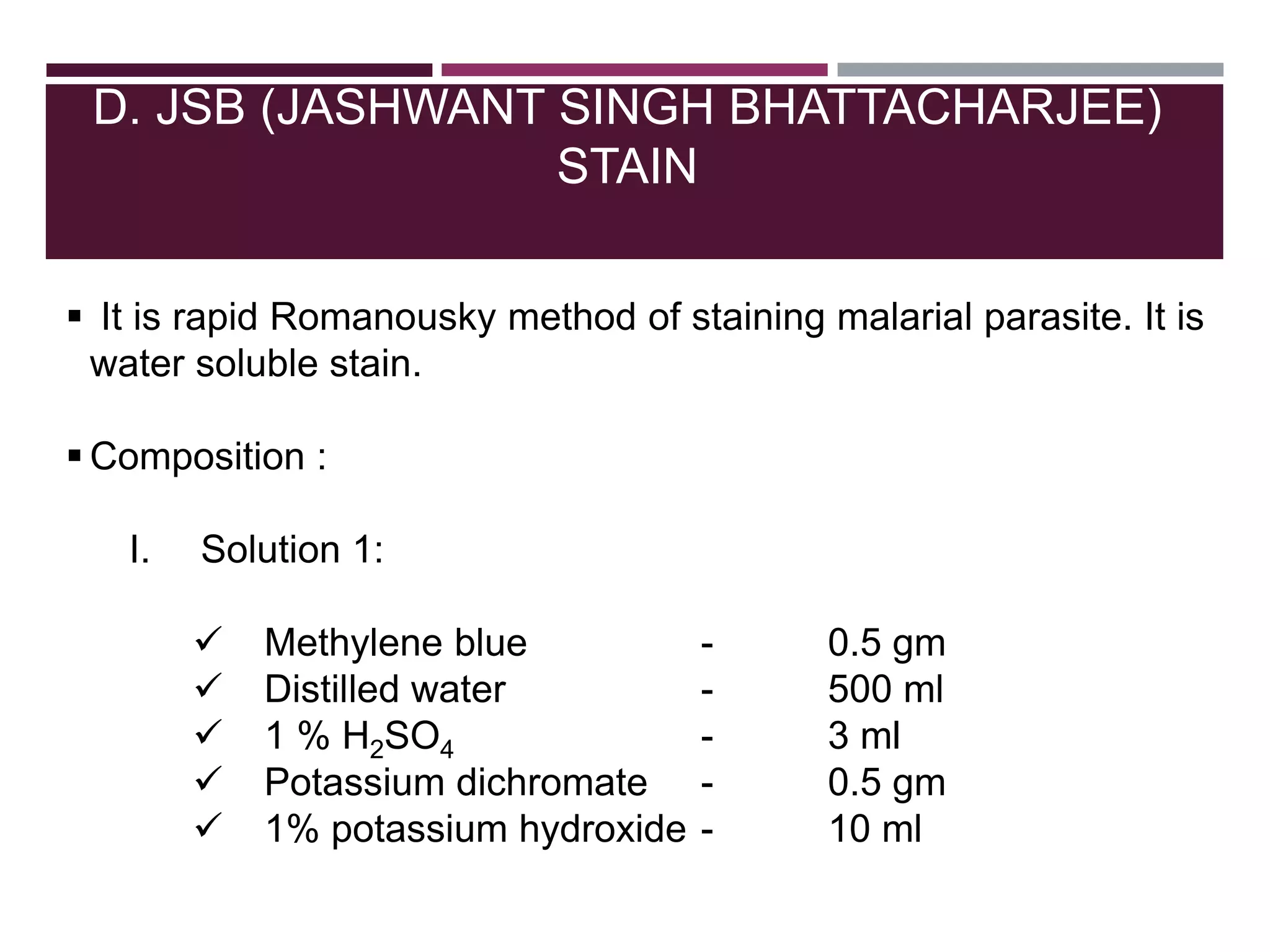
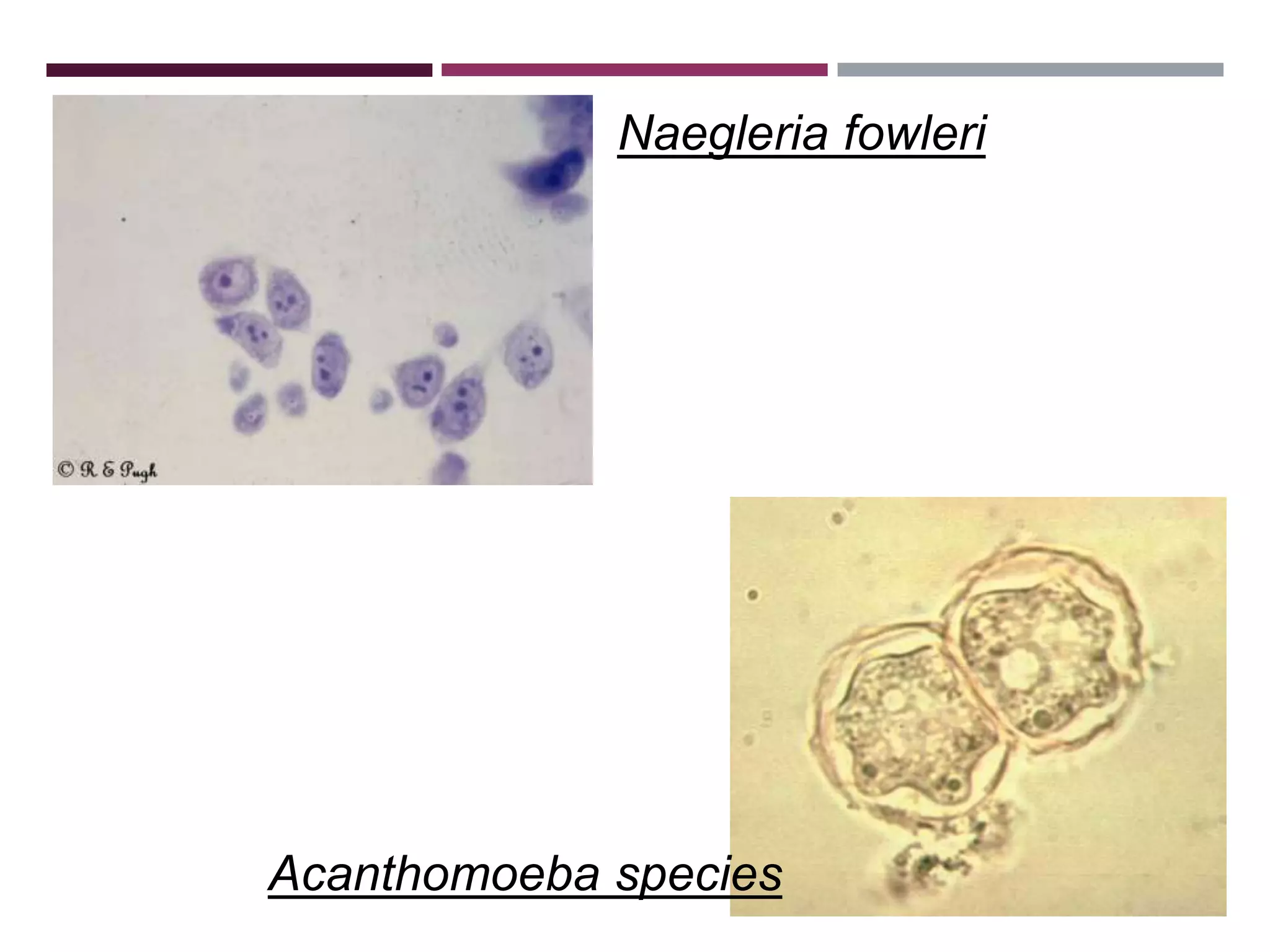
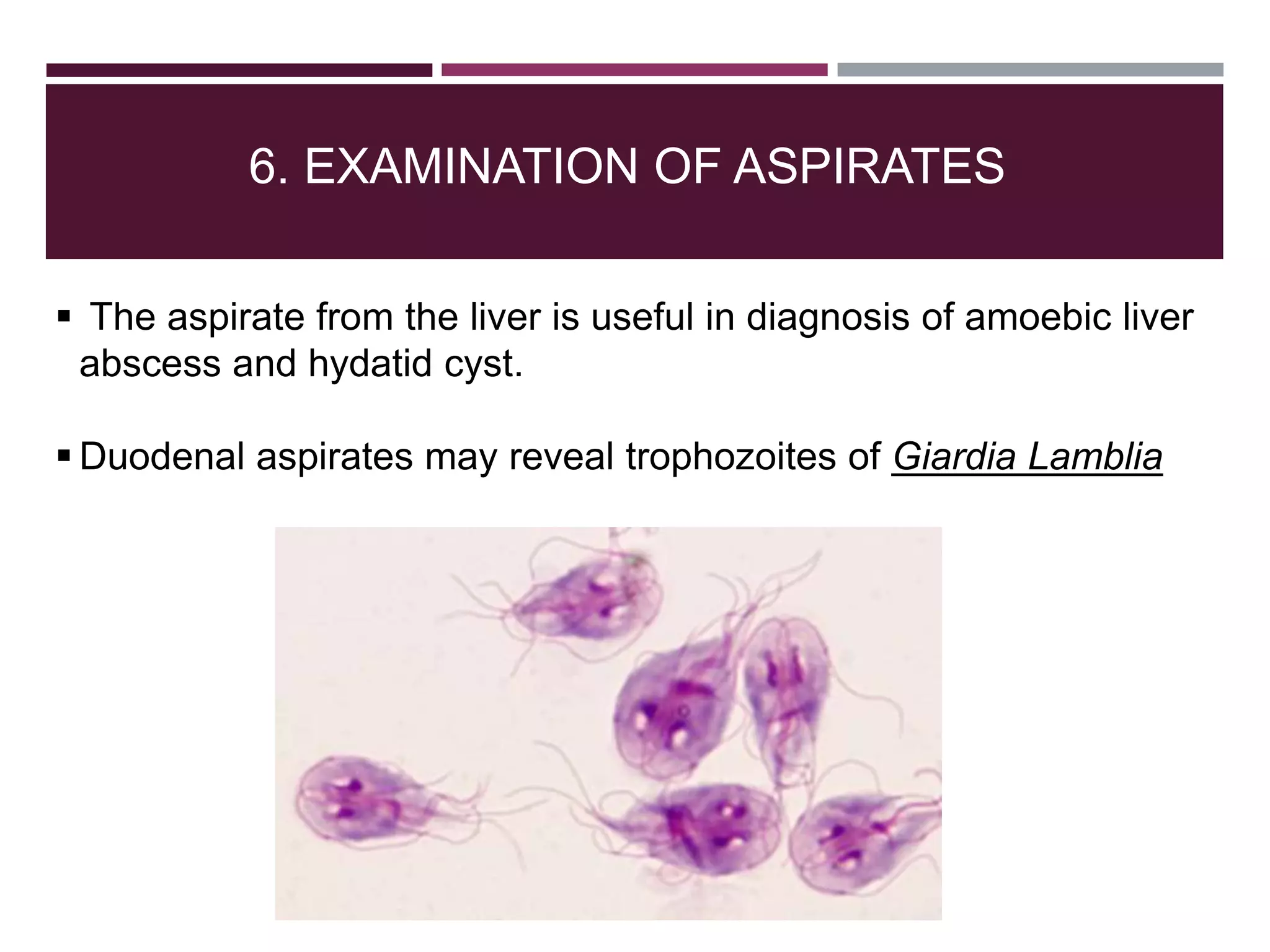
The document outlines various diagnostic methods for identifying parasites, emphasizing laboratory techniques for examining clinical specimens such as feces, blood, urine, and other bodily fluids. Key methodologies discussed include macroscopic and microscopic examination of feces, blood smear techniques, concentration methods, and serological testing, as well as the identification of specific parasites through staining and egg counting procedures. Each technique's procedures and considerations are detailed, focusing on accuracy and efficiency in diagnosing parasitic infections.