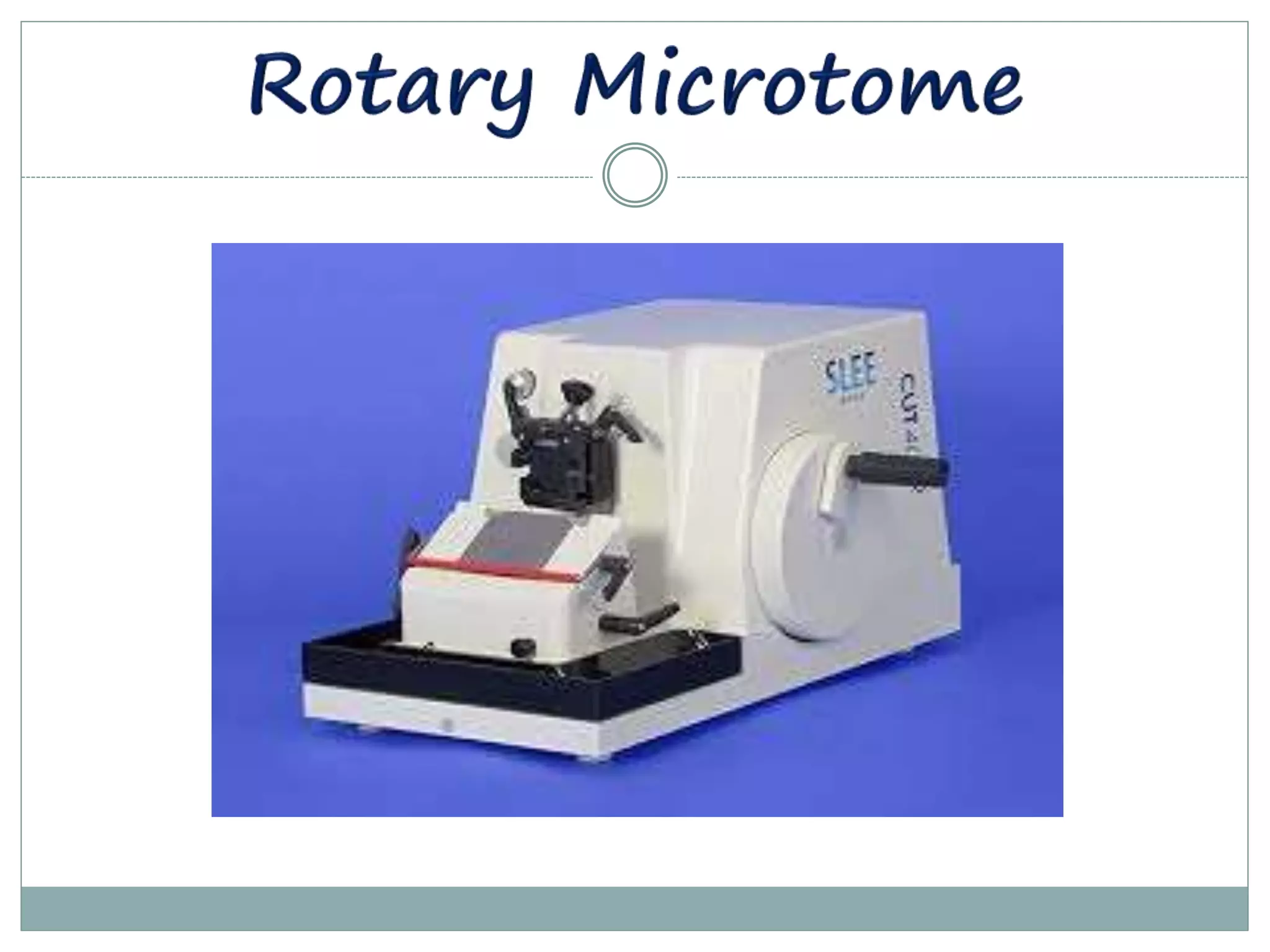
The document discusses cryostats, which are devices used to cut thin frozen sections of tissues for examination under a microscope. Cryostats contain a microtome inside a freezer unit that can rapidly freeze tissue samples and cut sections as thin as 1 micrometer at temperatures below freezing. The cryostat process allows for quick diagnosis by freezing and sectioning tissues within minutes rather than having to dehydrate, embed in paraffin, and section as with traditional microtomes.