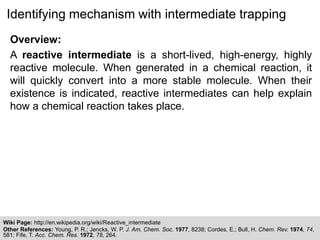
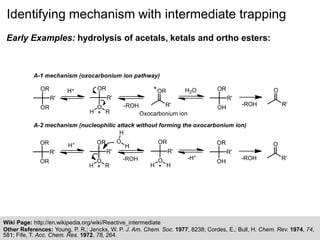
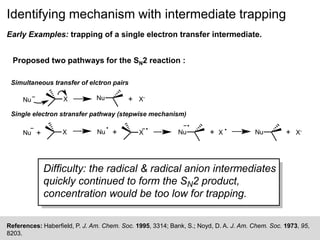
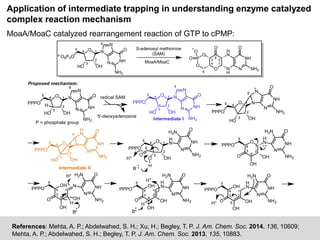
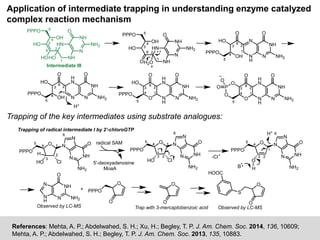
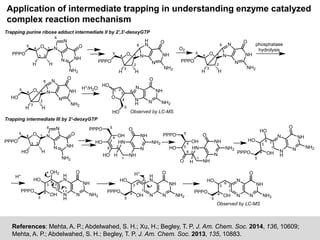
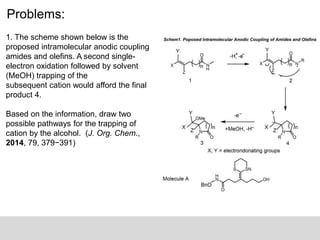
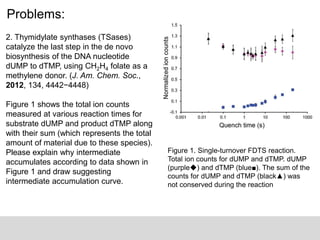
The document discusses the identification of reaction mechanisms using intermediate trapping of reactive intermediates, which are short-lived and highly reactive molecules. It covers examples involving the trapping of oxocarbonium ions and single electron transfer intermediates, as well as their implications in enzyme-catalyzed reactions. Furthermore, the authors propose pathways and applications of intermediate trapping using various substrates and techniques to understand complex reaction mechanisms in chemistry.
![Wiki Page: http://en.wikipedia.org/wiki/Reactive_intermediate
Other References: Young, P. R.; Jencks, W. P. J. Am. Chem. Soc. 1977, 8238; Cordes, E.; Bull, H. Chem. Rev. 1974, 74,
581; Fife, T. Acc. Chem. Res. 1972, 78, 264.
Trapping of the oxocarbonium ion intermediate by sulfite dianion:
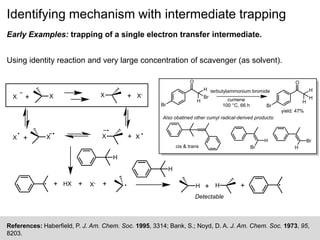
Identifying mechanism with intermediate trapping
• Results: achieved up to 97% trapping; rate constants for
acid-catalyzed cleavage of ketals are independent of
[SO3
2-] (Table 1).
• Conclusion: [SO3
2-] is not present in the TS of the RLS.
Reaction proceeds through the oxocarbonium ion
pathway.
H2O
R'
OR
Oxocarbonium ion
OH
OR
R'
R'
O
SO3
2-
SO3
-
OR
R'
Disulfide buffer
OR
OR
R'
Monitor absorbance change at 262 nm](https://image.slidesharecdn.com/decipheringreactionmechanismwithintermediatetrapping-170124154855/85/Deciphering-reaction-mechanism-with-intermediate-trapping-4-320.jpg)
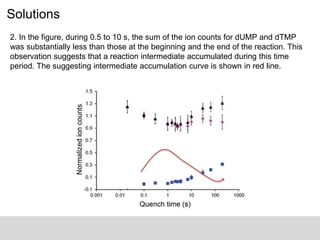
![Solutions
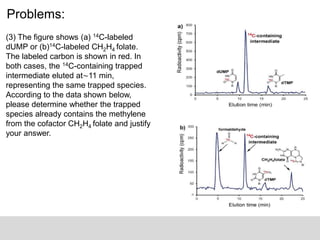
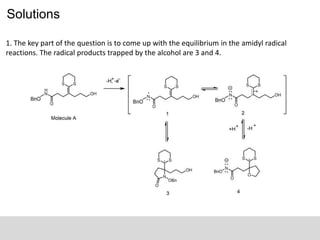
3. [11-14C] CH2H4 folate radiolabeled, a new radioactive peak had developed that
had the same retention time as the peak observed when starting with [2-14C]
dUMP. It means it is the same reactive intermediate. So it shows that the
intermediate nucleotide being chemically trapped during the acid quenching
had already undergone the condensation and that the carbon−carbon bond
between the C5 of dUMP and the methylene had been formed prior to the formation
of that intermediate.](https://image.slidesharecdn.com/decipheringreactionmechanismwithintermediatetrapping-170124154855/85/Deciphering-reaction-mechanism-with-intermediate-trapping-15-320.jpg)
