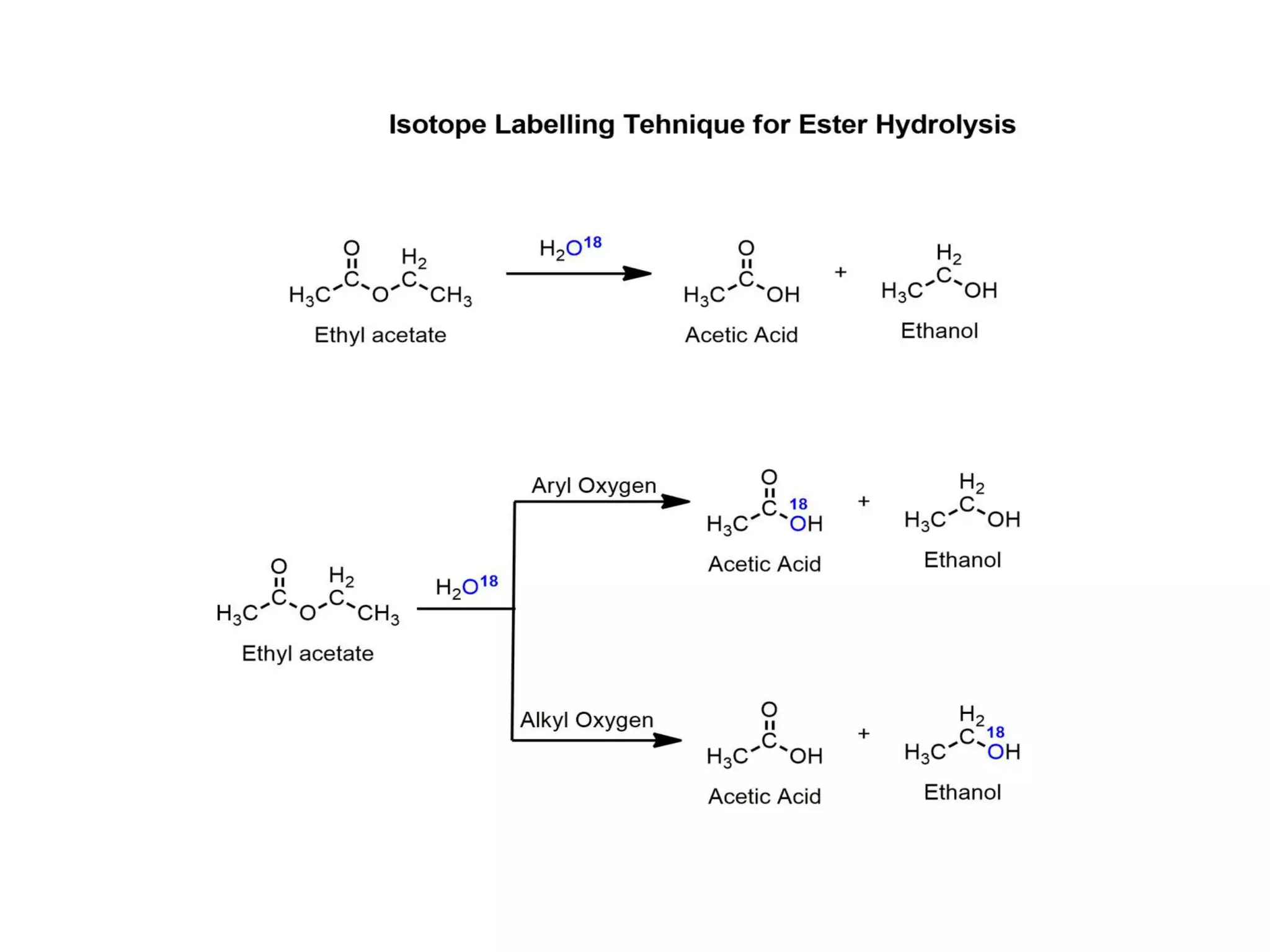
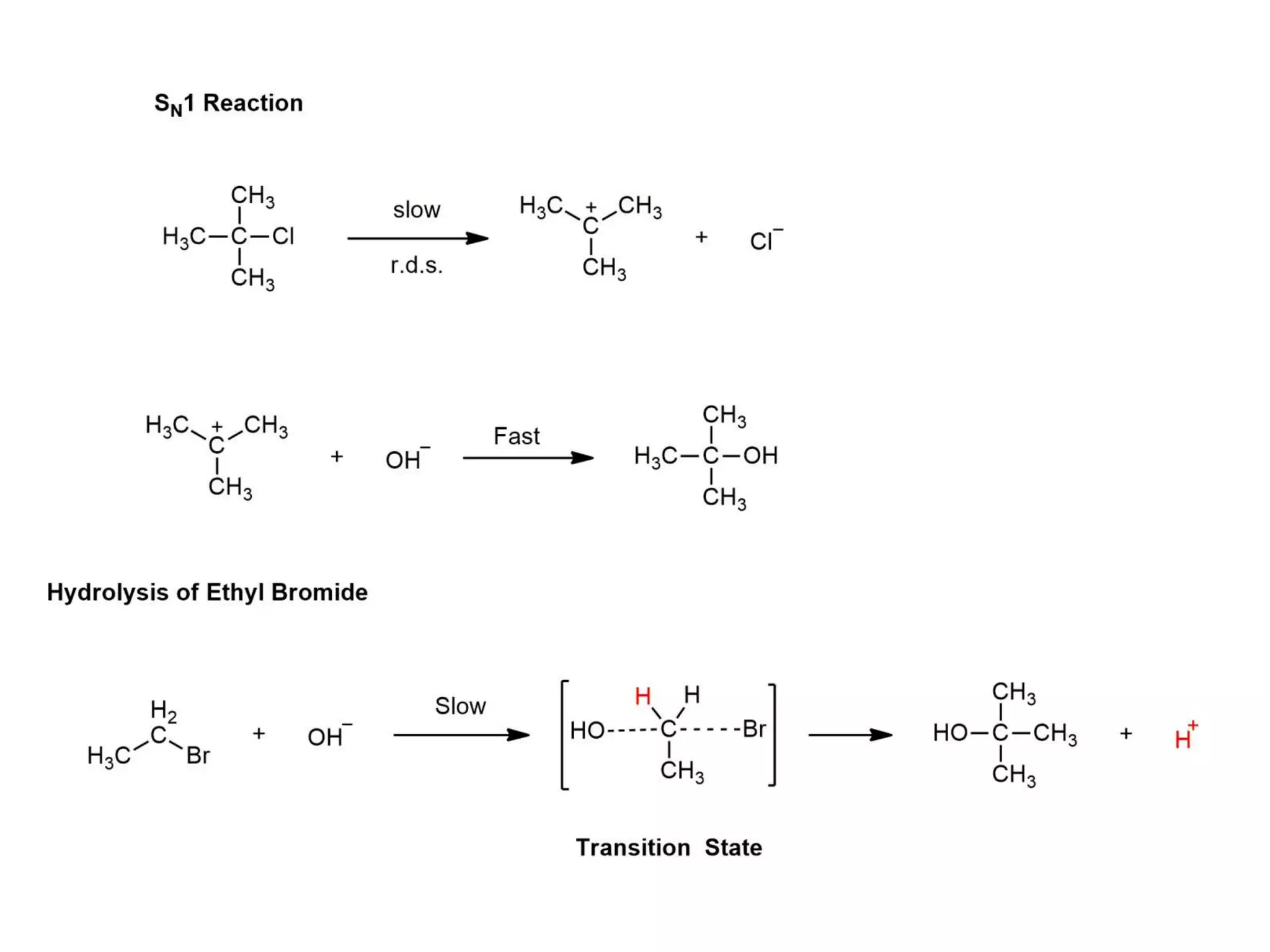
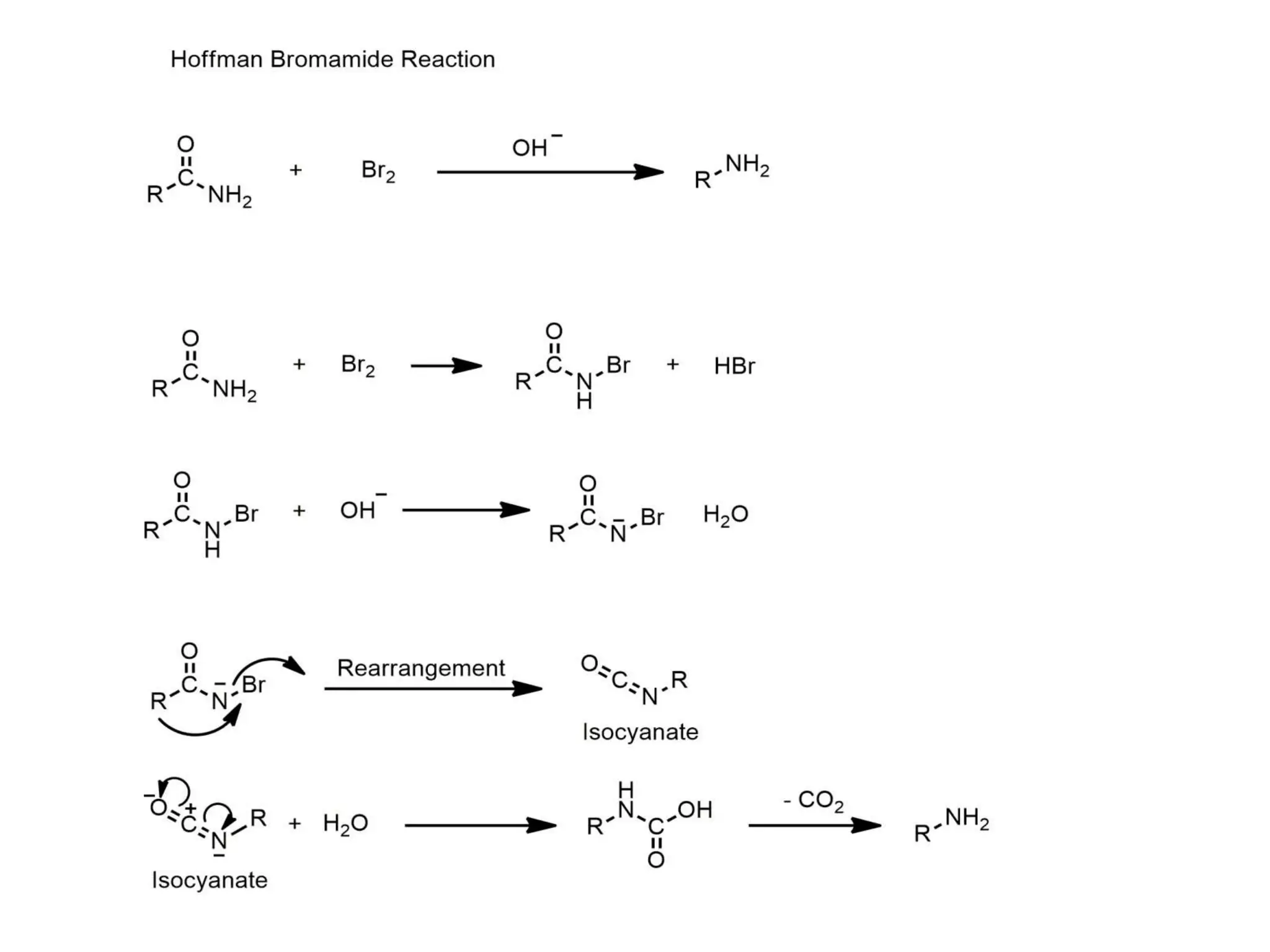
This document discusses various methods used to determine reaction mechanisms, including isotopic labeling techniques, stereochemical evidence, crossover experiments, identification of products, and identification of reaction intermediates. Isotopic labeling involves replacing atoms with isotopes like deuterium or carbon-13 to follow the reaction path. Stereochemical evidence from chiral reactants and products can indicate SN1 or SN2 mechanisms. Crossover experiments use non-identical reactants to study intramolecular vs intermolecular rearrangements. Identification of products and intermediates through spectroscopy, isolation, or trapping with reagents provides clues about reaction steps.