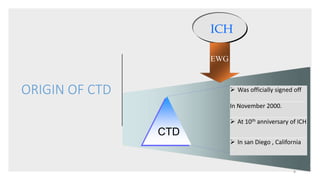
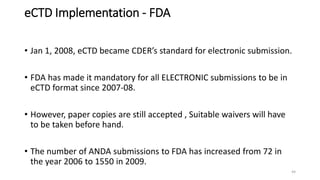
The document provides information on the Common Technical Document (CTD) format for organizing technical documents submitted to regulatory authorities for approval of pharmaceutical products. The CTD format was developed by the International Conference on Harmonization to streamline review processes and facilitate simultaneous submissions across different regions. It includes five modules covering administrative information, summaries, quality, nonclinical and clinical data. Adopting a common format provides benefits like reduced submission time and costs as well as faster availability of new medicines to patients.