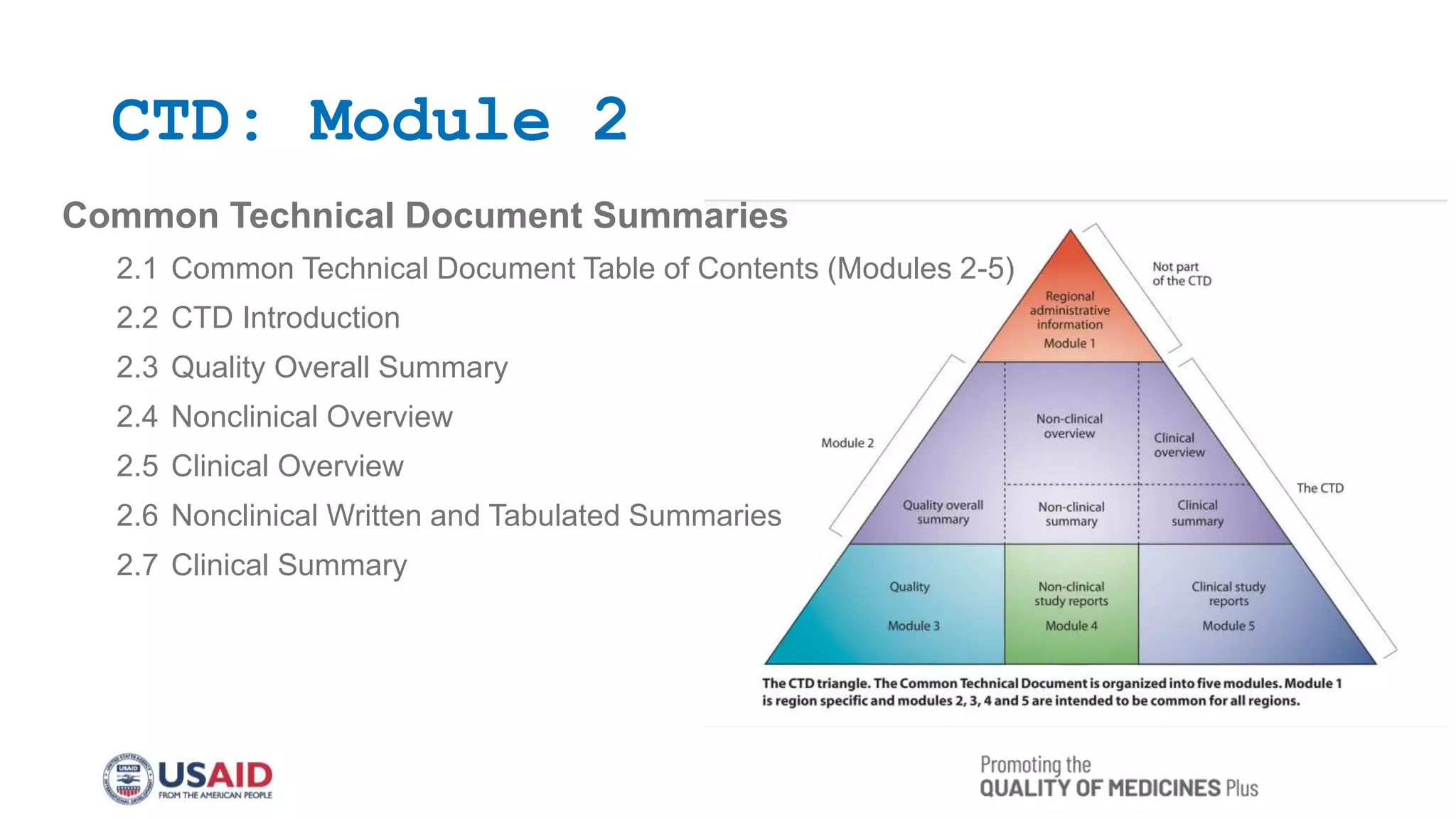
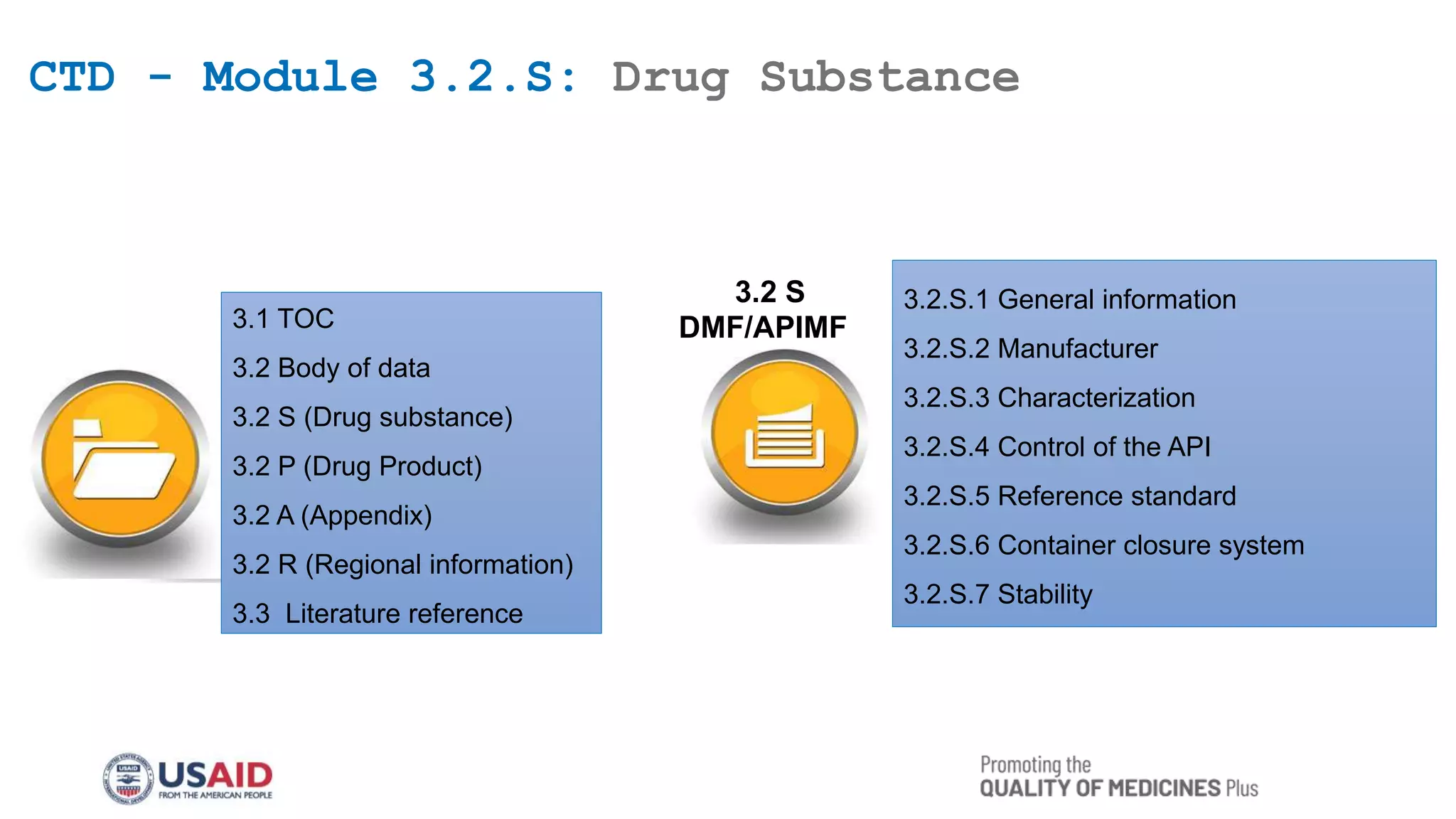
This document outlines the Common Technical Document (CTD) guidelines established by the International Council for Harmonization (ICH) for the submission of pharmaceutical applications. It details the structure of the CTD, which consists of five modules, including regional specifics in Module 1 and common standards in Modules 2 to 5, aimed at harmonizing the regulatory process worldwide. The guidelines seek to reduce the time and resources needed for application preparations and to facilitate regulatory reviews through standardized documentation.