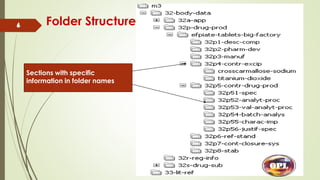
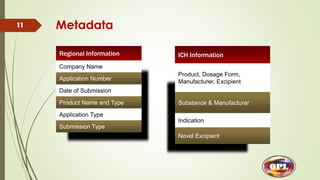
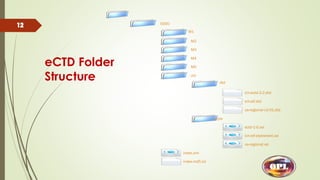
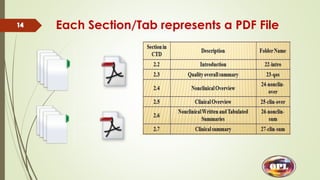
This document provides an overview of the electronic Common Technical Document (eCTD) format used for regulatory drug submissions. It discusses the history and goals of the ICH and eCTD, the components and structure of an eCTD, best practices for preparing documents, and software options. Key points covered include the folder structure, use of XML and metadata, concept of reuse and granularity, and comparing the benefits of eCTD to traditional paper submissions. The conclusion emphasizes that adopting eCTD is essential to joining the electronic bandwagon, while also needing intermediate steps to fully transition from paper CTD formats.