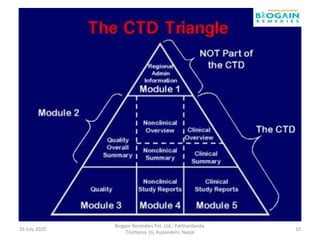
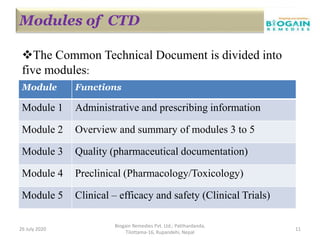
The document outlines the preparation of a Common Technical Document (CTD) for pharmaceutical product registration, detailing the requirements and structure for marketing authorization applications in various regions. It explains the specific modules of the CTD, including administrative information, quality data, and clinical trial results necessary for proving the drug's safety and efficacy. Additionally, it highlights the transition from paper CTD to the electronic Common Technical Document (eCTD), emphasizing improved processes and information management.