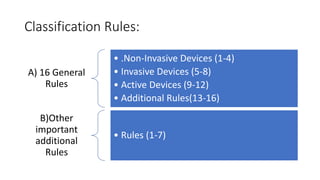
This document summarizes the objectives and classification system of the Global Harmonization Task Force (GHTF) for in vitro diagnostic (IVD) medical devices. The GHTF was founded in 1993 to harmonize medical device regulations globally. It aims to facilitate trade while preserving public health. IVD medical devices are classified into 4 risk-based classes (A to D) based on 16 general rules related to device invasiveness, energy use, and disease detection. Class A devices pose the lowest risk while Class D the highest. The classification system aims to ensure regulatory oversight is proportionate to device risk.