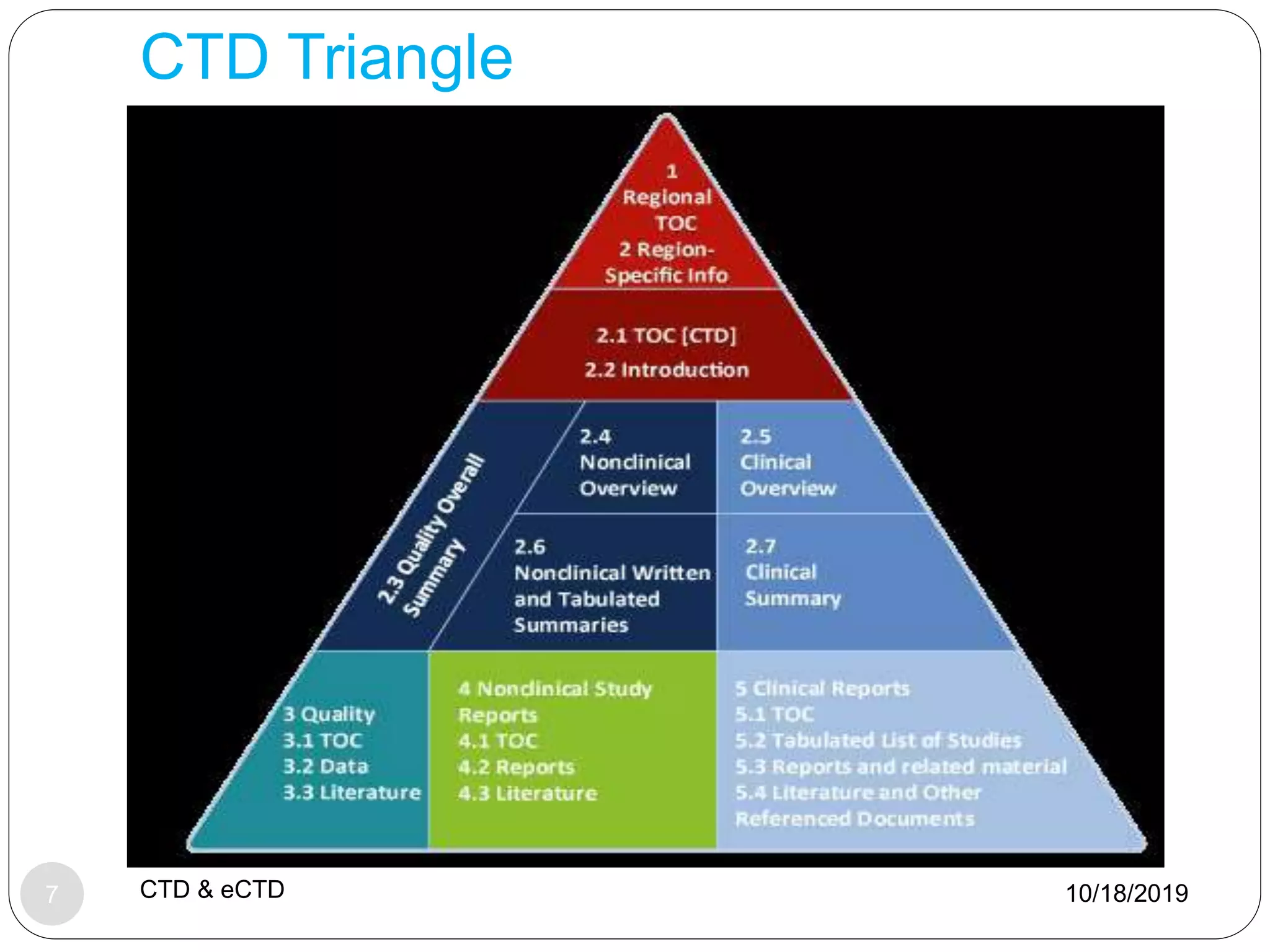
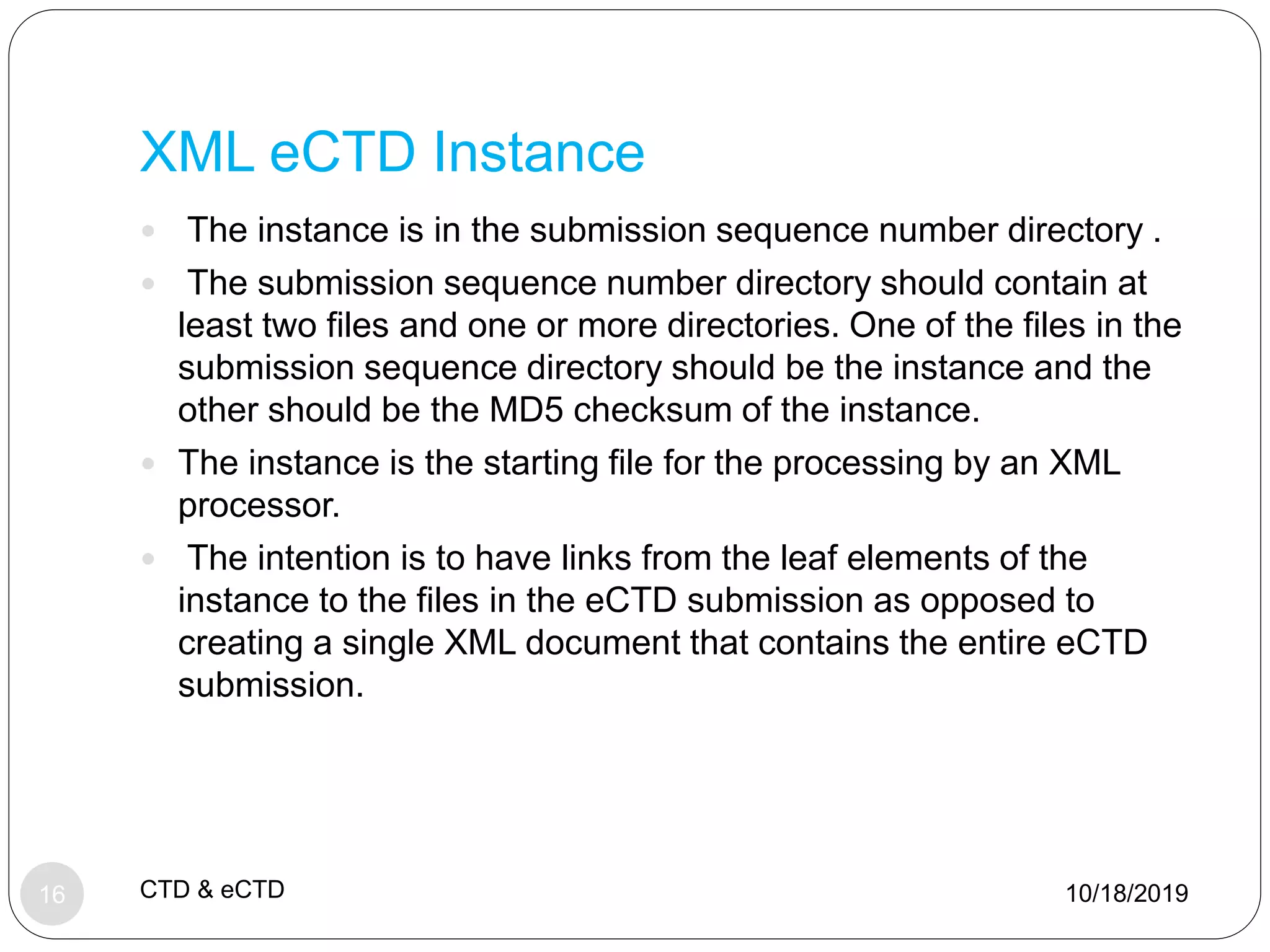
The document discusses the Common Technical Document (CTD) format and electronic CTD (eCTD) format for new drug applications. The CTD format was agreed upon in 2000 and standardized the organization of application modules for quality, nonclinical, and clinical information. The eCTD is the electronic equivalent that regulatory agencies now require for application submissions. Key benefits of the eCTD include increased efficiency, reduced costs, and improved review processes through electronic submission and evaluation of drug applications.