The document discusses the Common Technical Document (CTD), which is a standardized format for submitting regulatory information to the FDA, EMA, and MHLW. It provides an overview of the CTD, including its evolution, modules, advantages, and comparison to the electronic CTD format. The key points are:
1) The CTD is a standardized format adopted by the FDA, EMA and MHLW to streamline the submission and review of documentation for drug approval.
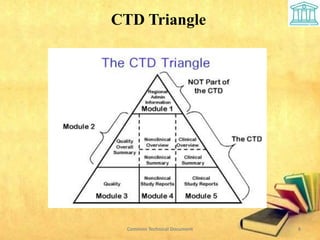
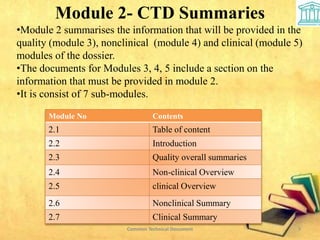
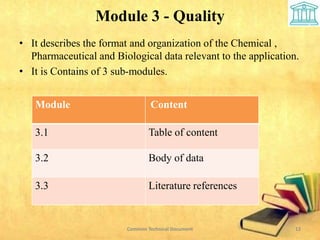
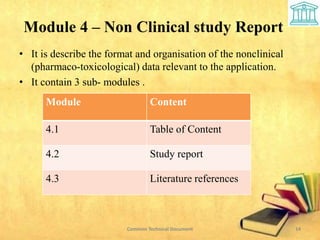
2) It is divided into 5 modules covering administrative information, summaries, quality, nonclinical and clinical data.
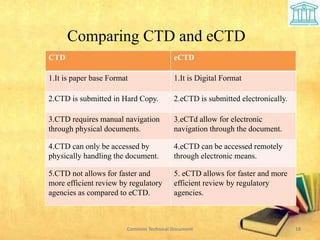
3) The electronic CTD (eCTD) allows for digital submission and navigation, remote access and faster