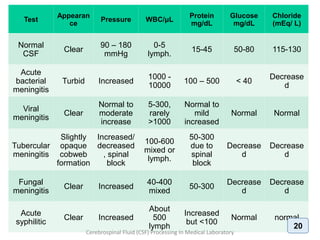
1. The document discusses the procedures for examining cerebrospinal fluid (CSF) samples in a medical laboratory, including gram staining, cell counting, and culture. Gram staining should be performed first to detect the presence of bacteria or yeast. A cell count is also required to determine the type and number of white blood cells present. CSF samples showing signs of infection based on gram stain or cell count should be cultured. The goal is to rapidly diagnose conditions like bacterial or fungal meningitis.