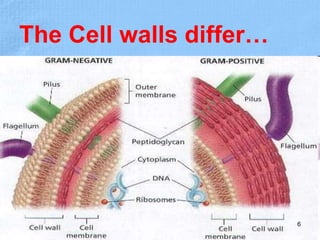
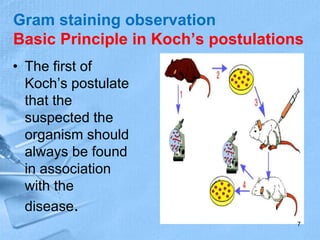
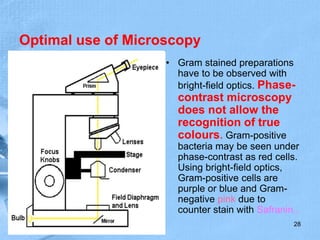
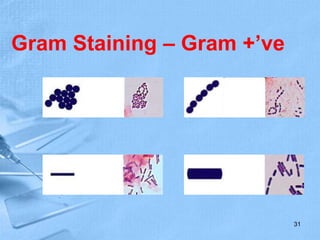
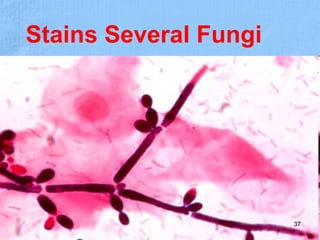
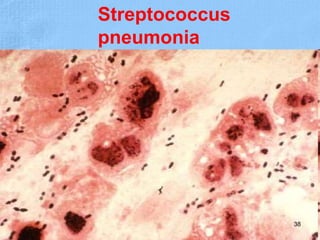
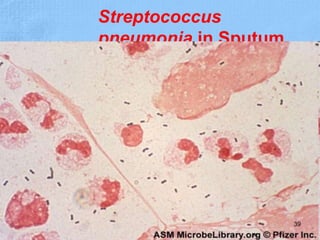
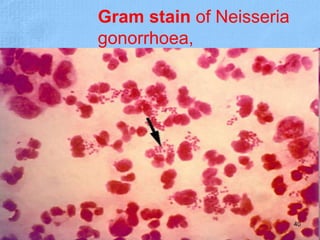
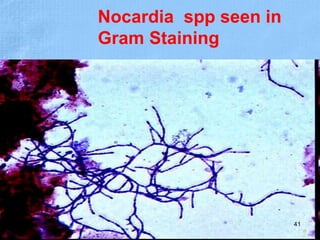
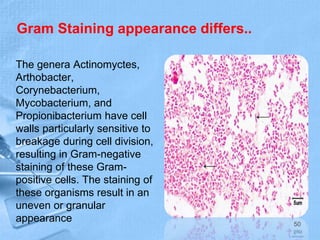
The document provides details about the history and procedure of Gram staining. It discusses how Hans Christian Gram developed the staining technique in 1883 while studying pneumonia. It was later modified by Carl Weigert who added a safranin counterstain. The traditional Gram staining procedure involves staining with crystal violet, iodine, decolorizing with alcohol, and counterstaining. Gram staining differentiates bacteria as either gram-positive or gram-negative based on differences in cell wall structure and composition. The document outlines the detailed steps and considerations for performing Gram staining accurately.