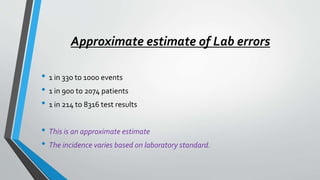
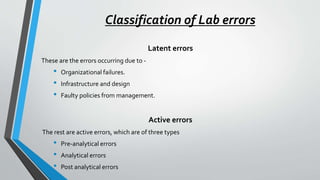
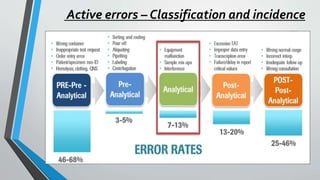
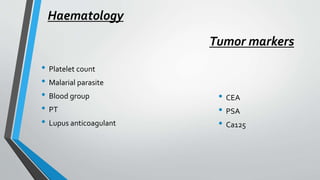
The document discusses laboratory errors, defining them as misdiagnoses or defects throughout the laboratory process, and provides estimates of their incidence. It emphasizes the importance of addressing complaints and internal audits to identify and mitigate both latent and active errors, while outlining preventive actions and protocols to enhance accuracy and reliability in laboratory results. The aim is continuous improvement in quality management to ensure better patient care.