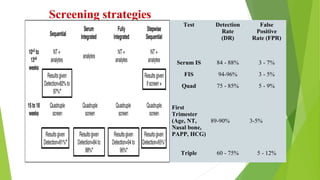
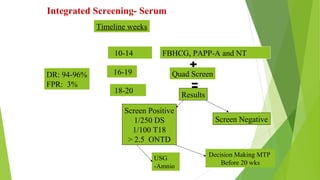
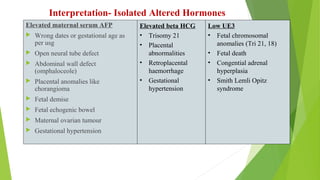
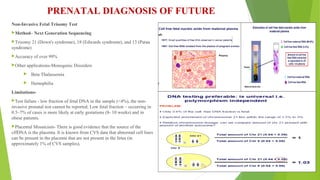
The document outlines the importance of maternal screening for genetic abnormalities and congenital diseases, highlighting statistical risks for conditions like trisomy 21, 18, and neural tube defects in the Indian population. It discusses the rationale for prenatal screenings, recommended tests, and the significance of hormonal measurements in assessing pregnancy risks. Additionally, it covers the reliability and limitations of non-invasive prenatal testing methods, along with screening for pre-eclampsia.






![Laboratory QC Criteria
Alpha-Fetoprotein [AFP] Assay
• The coefficient of variation should not exceed 5%. The
accuracy should be within 3% from lot to lot.
Chorionic Gonadotropin [hCG] Assay
• The coefficient of variation should not exceed 5%. The
accuracy should be within 3% from lot to lot.
Unconjugated Estriol [uE3], PAPP-A, Free B-HCG Assay
• The coefficient of variation should not exceed 7%. The
accuracy should be within 5% from lot to lot.
-Monthly monitoring of individual parameter MOM
- Monthly monitoring of positivity rates for T21, T13/18,
NTD
- Quarterly monitoring of individual parameter MOM in
comparison with shared database, there by updating the
MOM as per Indian population](https://image.slidesharecdn.com/maternalscreening-190301145423/85/Maternal-screening-for-fetal-Aneuploidy-Update-on-Laboratory-Tests-11-320.jpg)