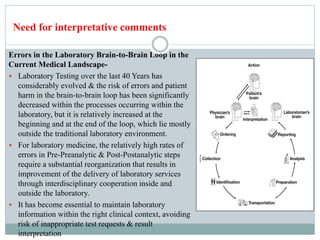
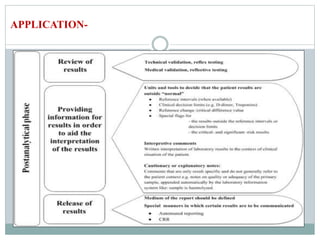
The document discusses the importance of interpretative comments in laboratory reports, emphasizing their role in improving clinical decision-making and patient safety by aiding in the accurate interpretation of complex laboratory data. It highlights the necessity for such comments due to evolving diagnostic tests, the inadequacy of medical education on laboratory test interpretation, and the need to prevent diagnostic errors. Furthermore, it outlines challenges and concerns regarding the implementation of interpretative comments, advocating for their incorporation to enhance the quality of laboratory services.