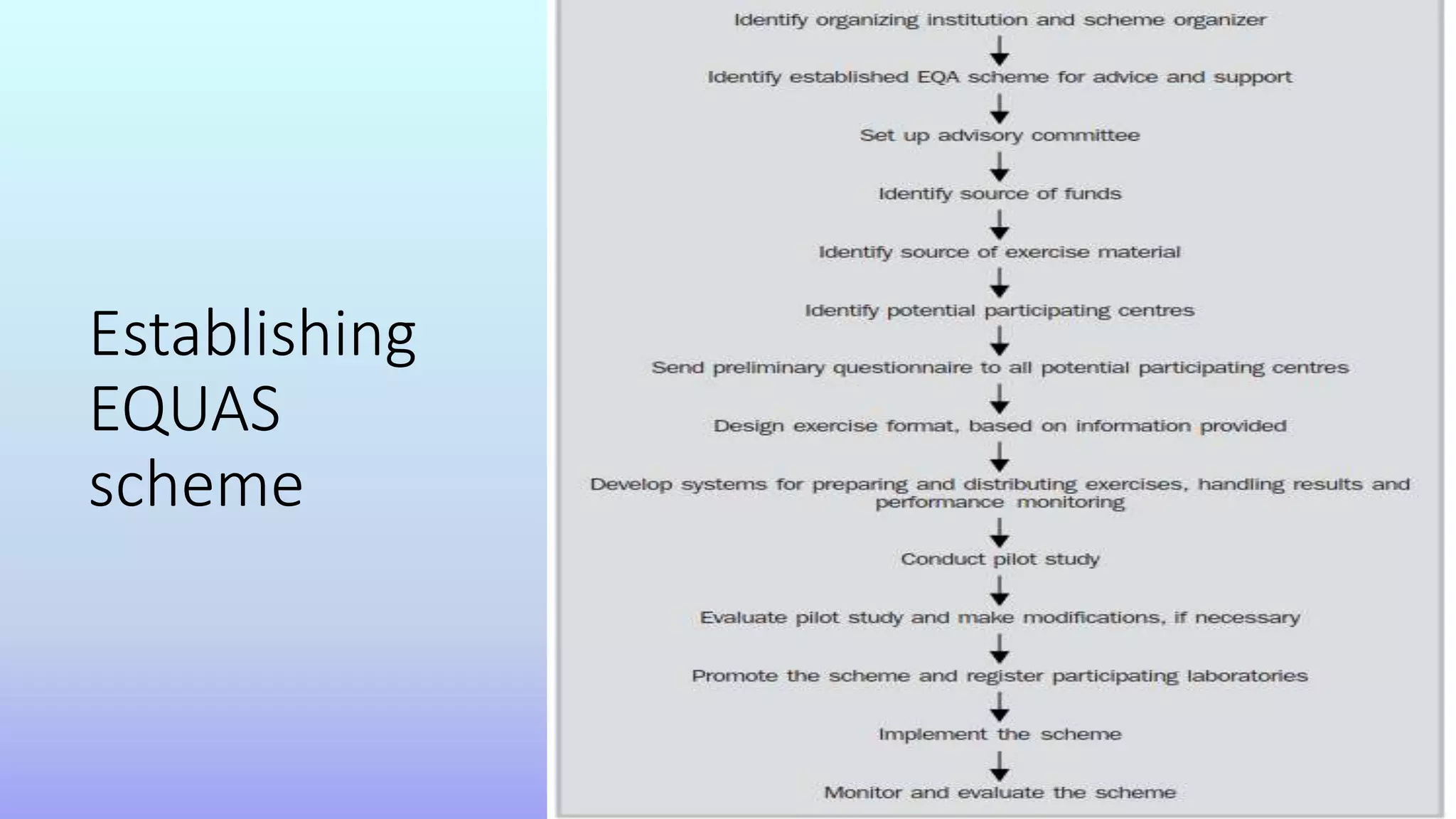
This document discusses external quality assurance (EQA) of serological testing. It outlines key elements of a quality system including documentation, training, assessment, and standards. EQA involves laboratories testing unknown samples provided by an EQA scheme and comparing results to improve accuracy. Participating in EQA allows laboratories to identify any issues, enhance performance, and ensure quality standards are met through objective review of results across laboratories. EQA schemes provide benefits for laboratories and regulatory authorities by establishing networks to improve testing practices and public confidence in testing standards.