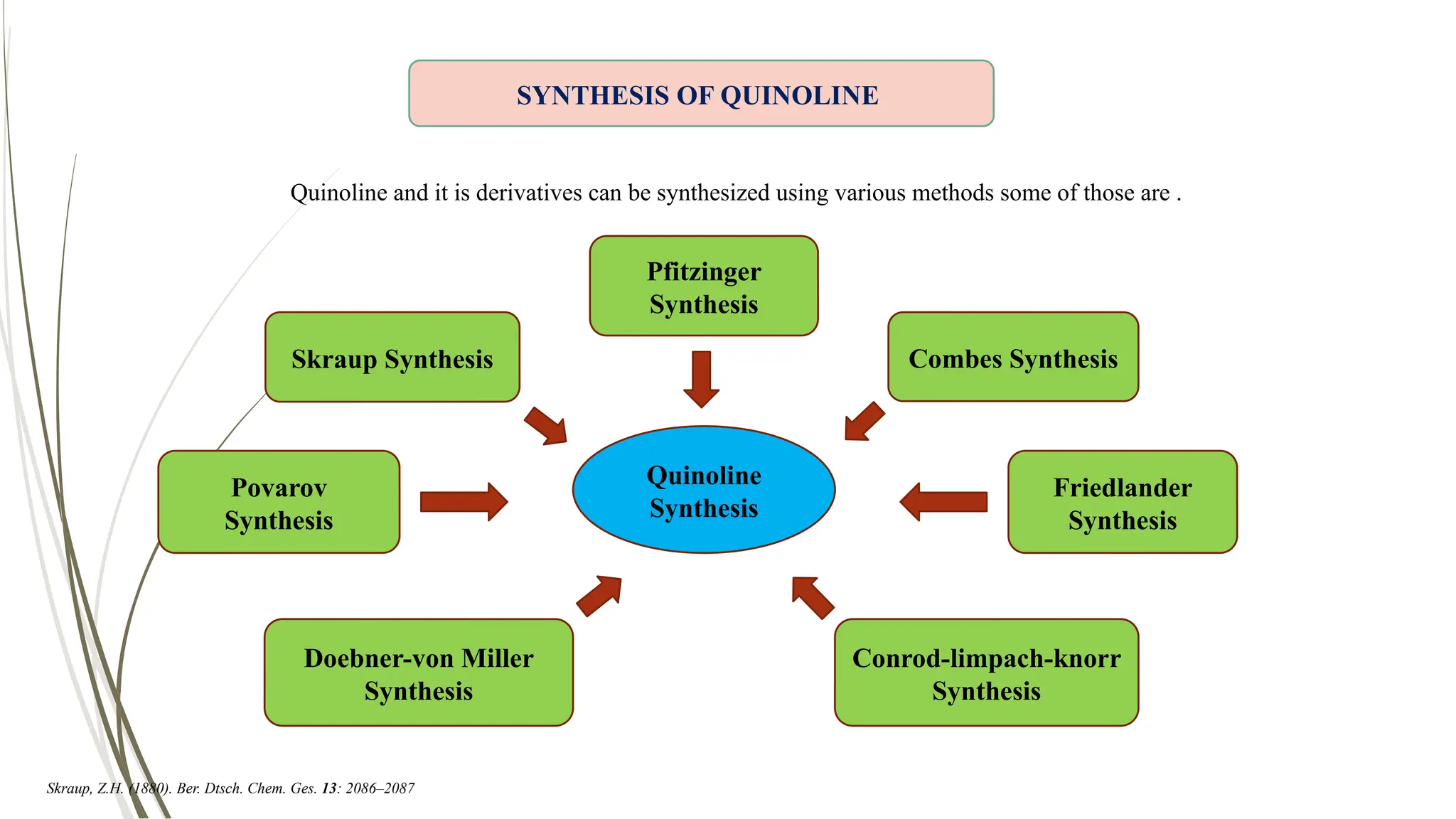
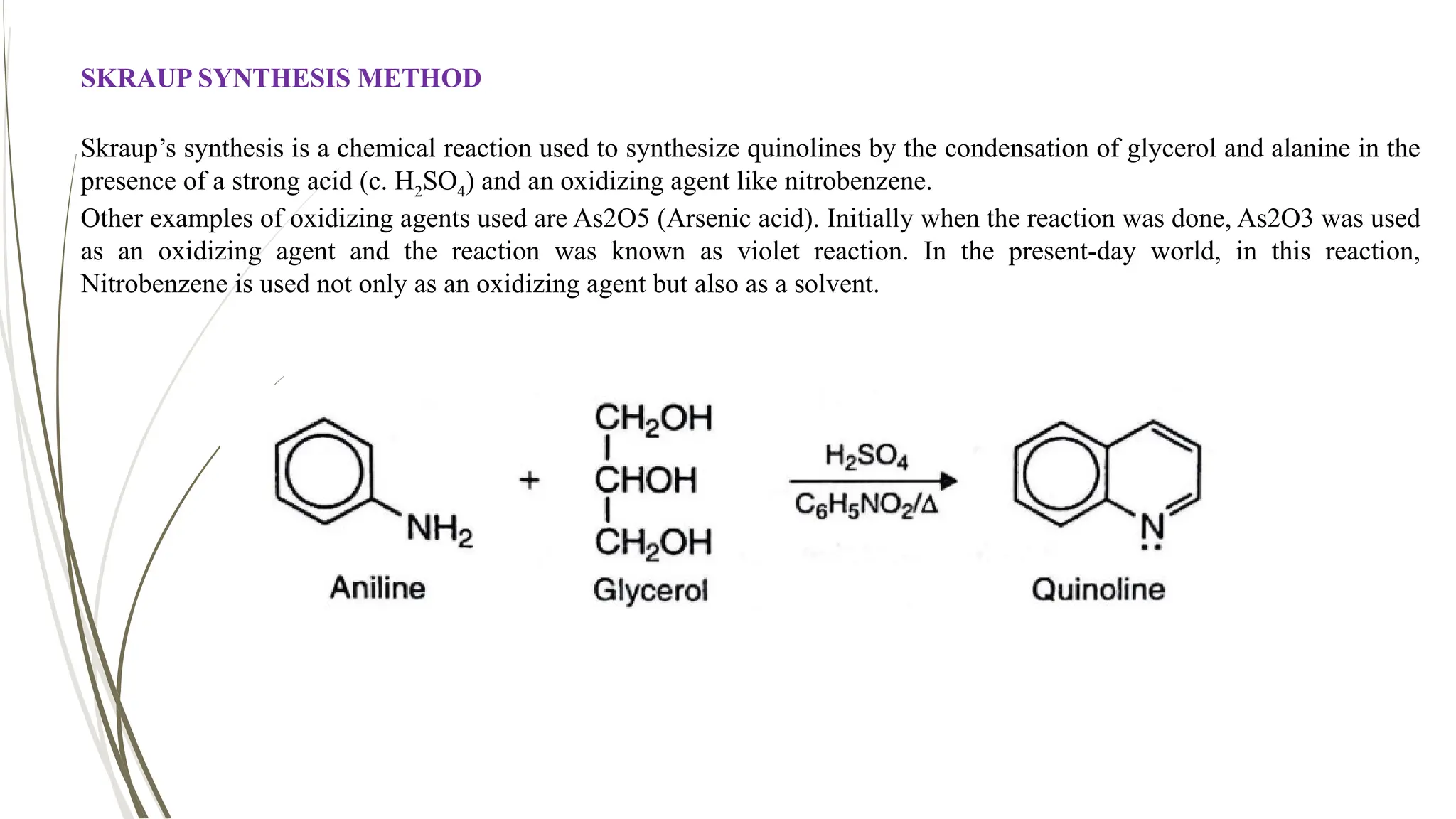
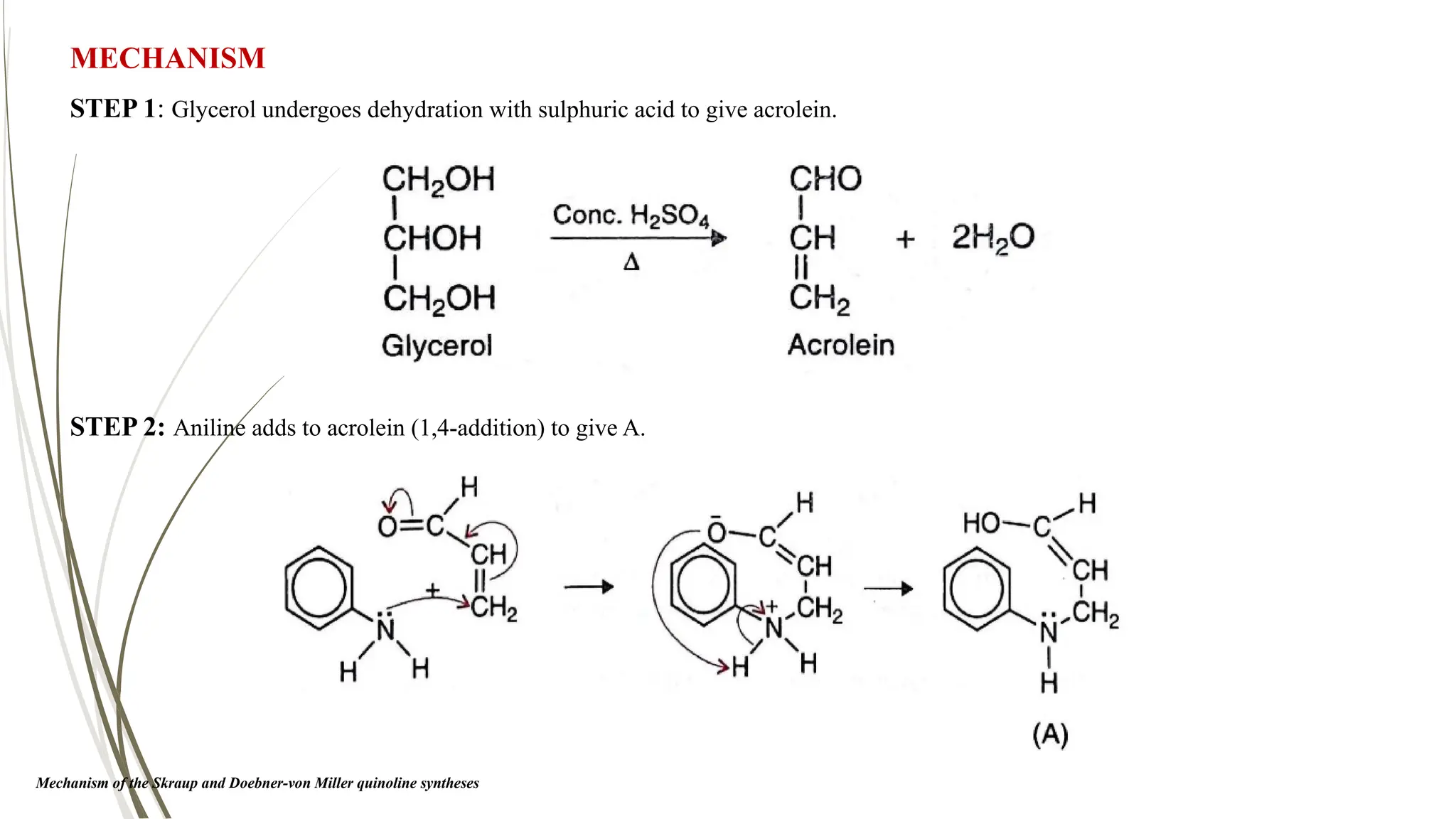
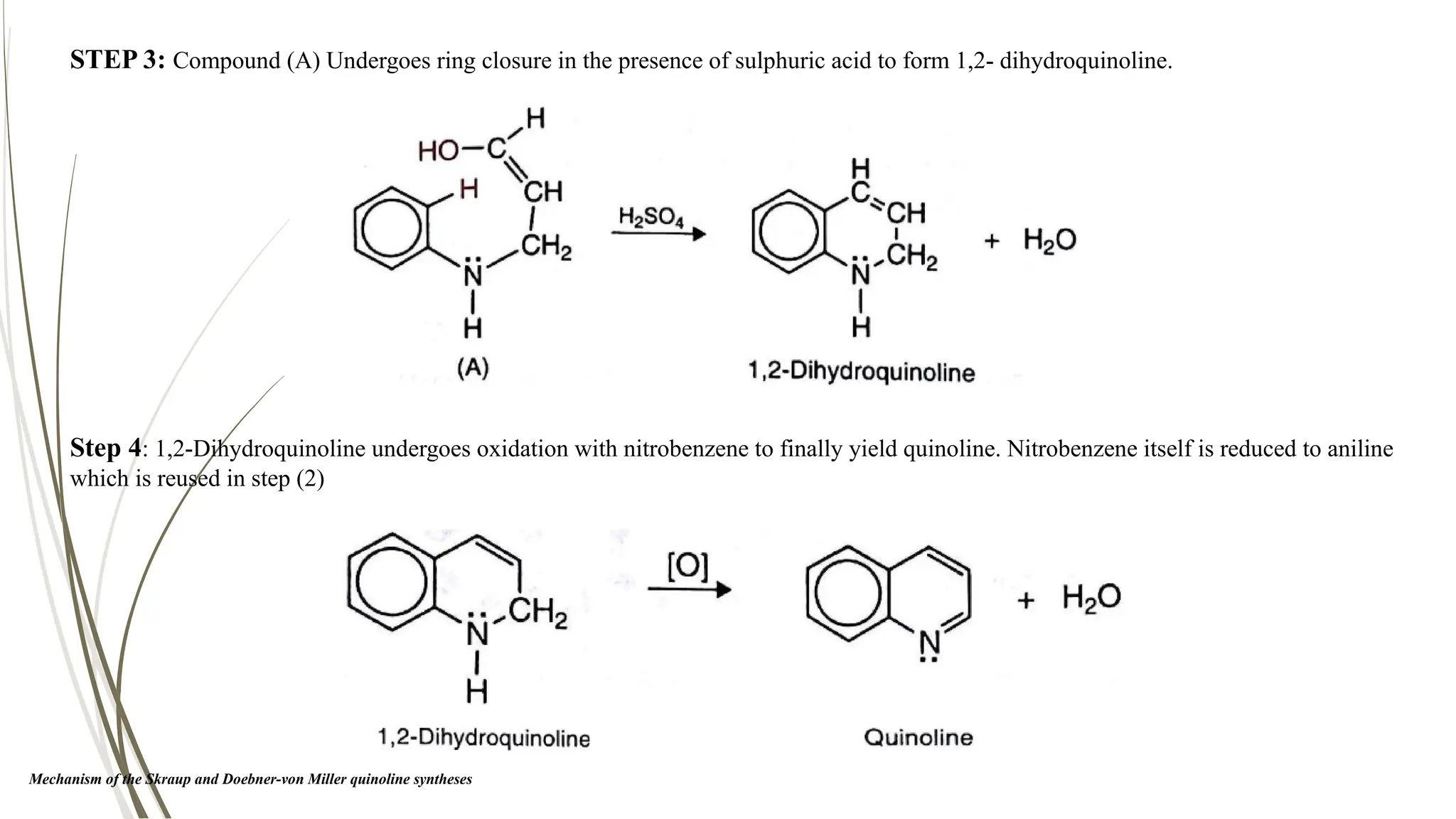
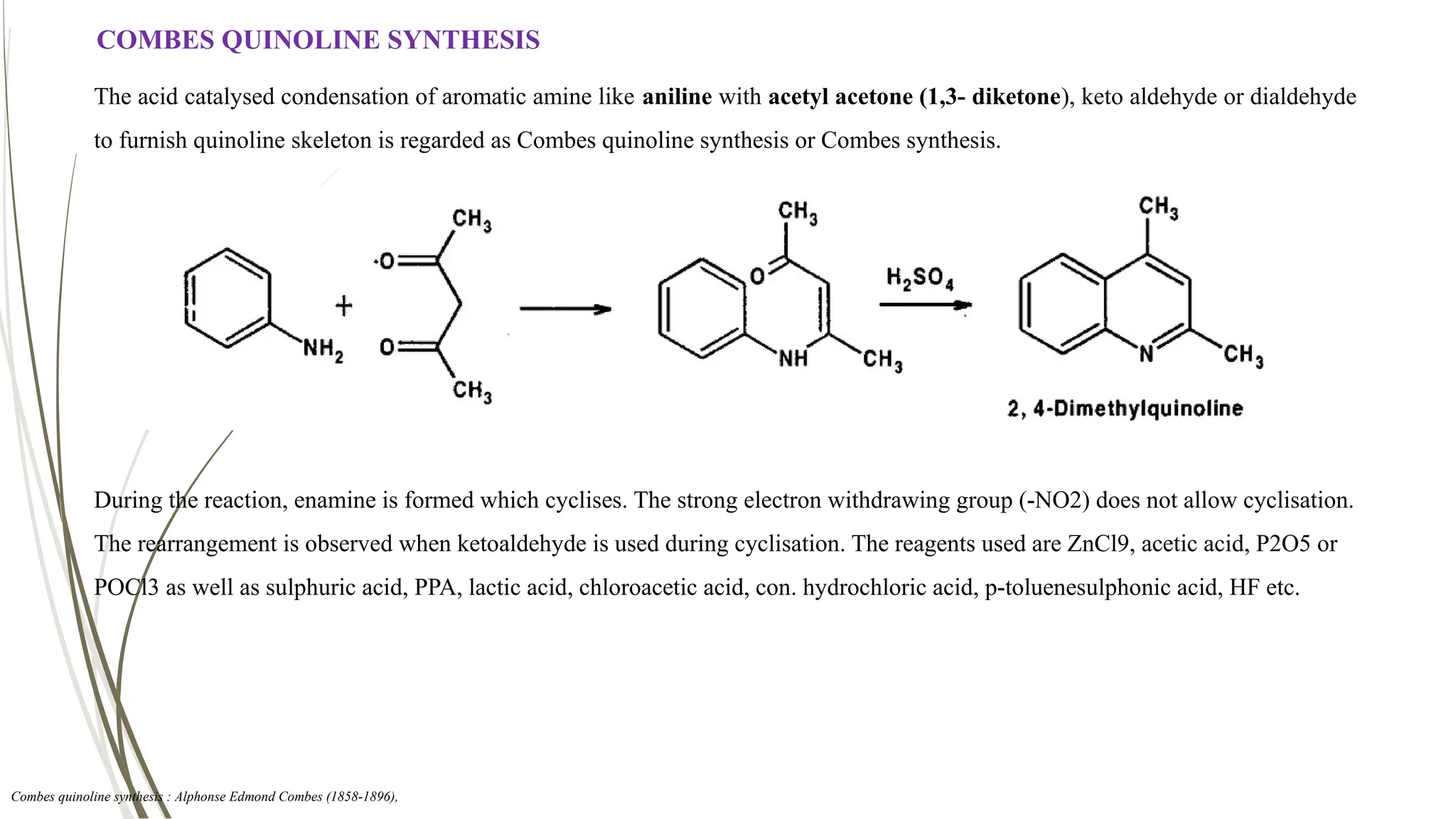
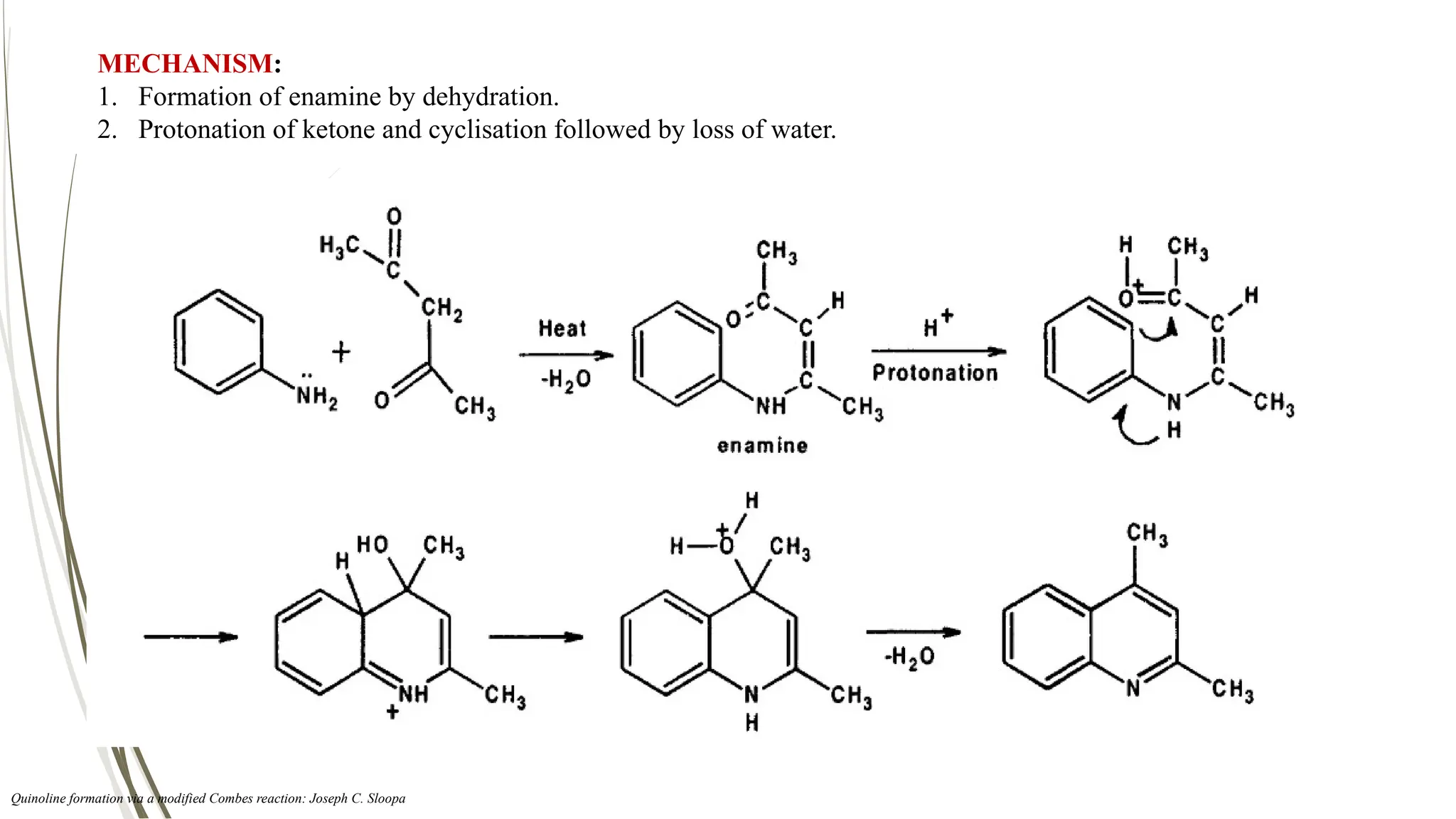
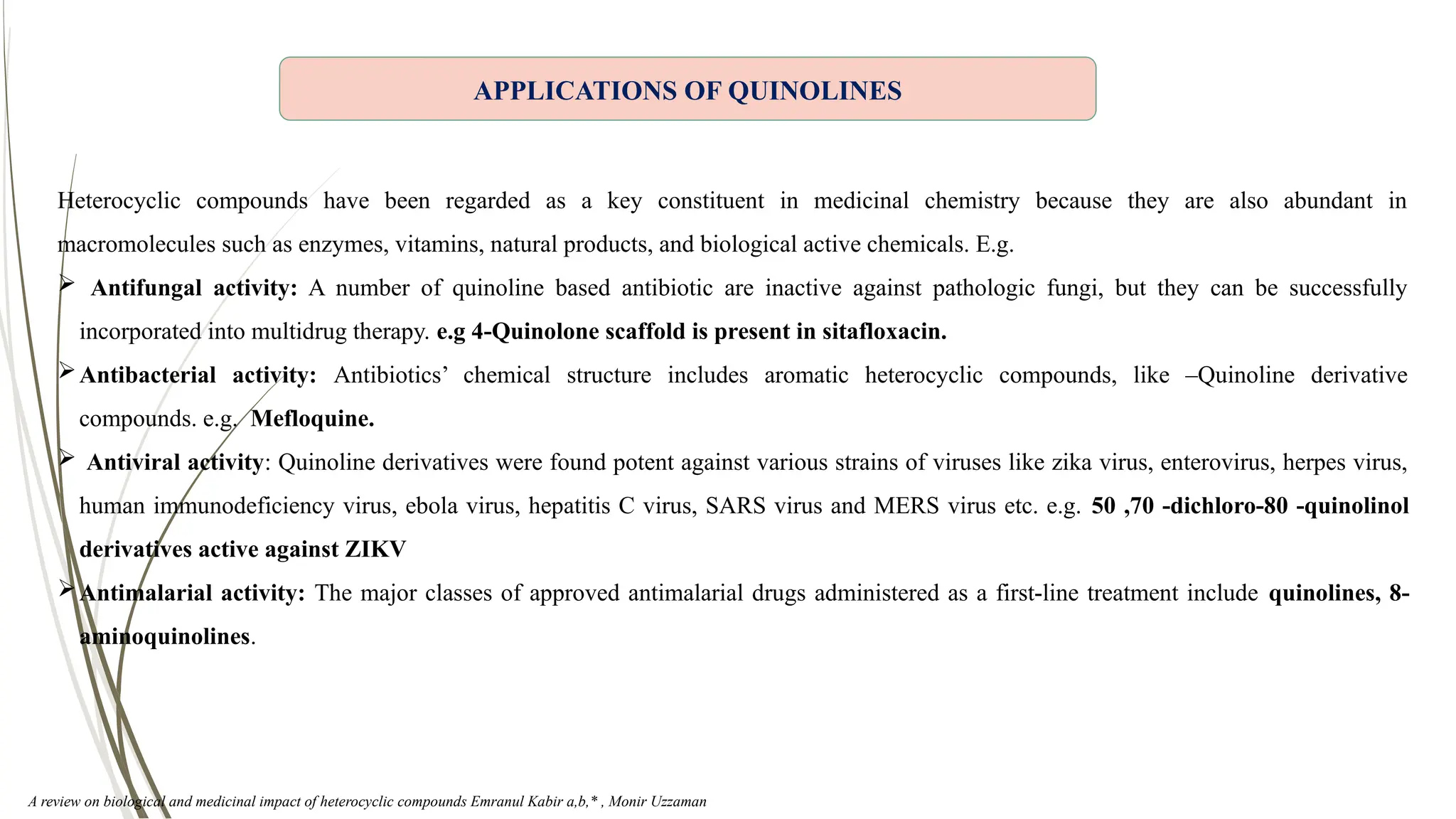
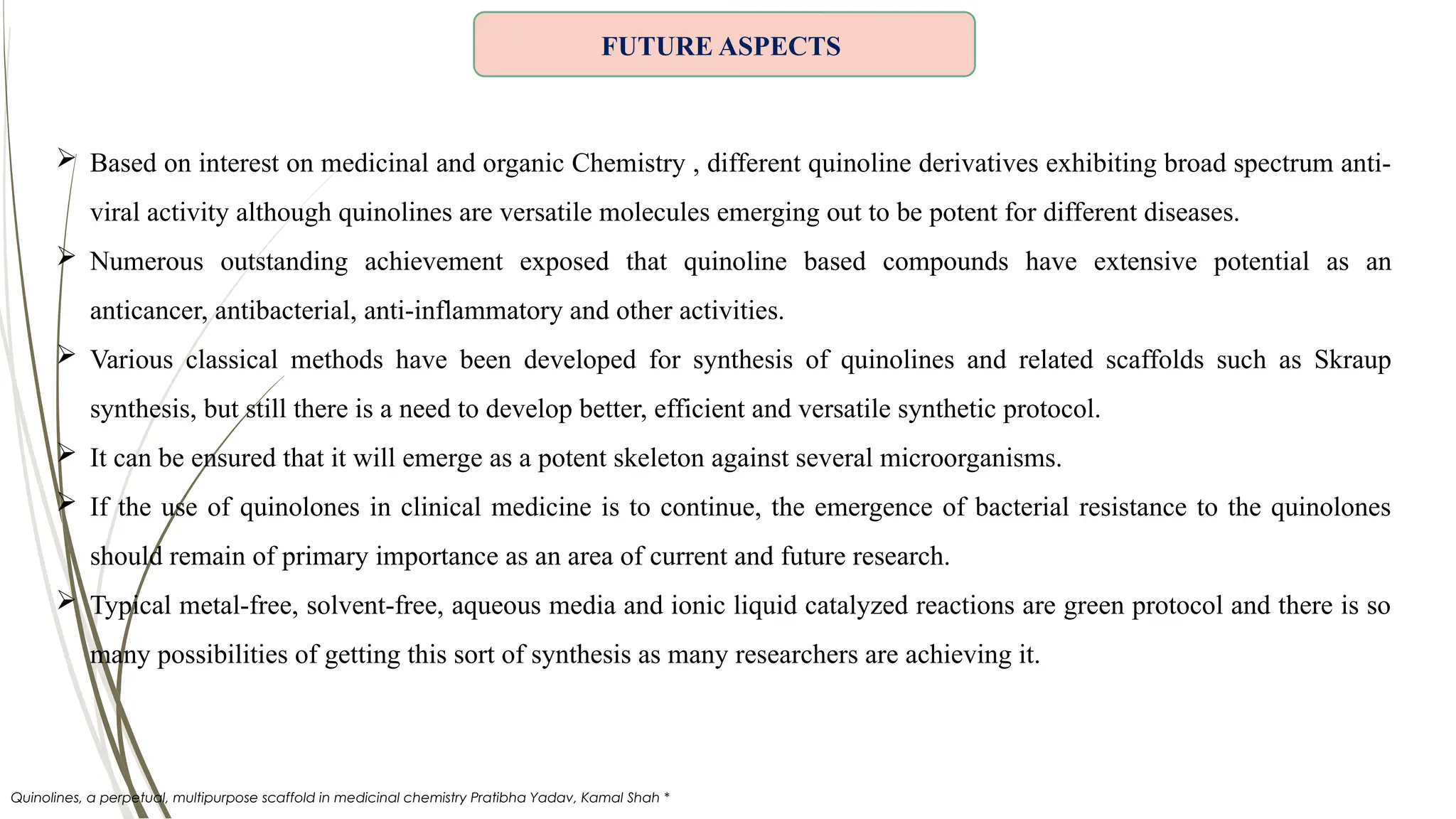
The document discusses the synthesis and applications of quinoline derivatives, emphasizing their significance in medicinal chemistry and various biological activities such as antibacterial and antiviral properties. It highlights various synthetic methods, including Skraup and Combes reactions, and the need for improved synthesis protocols. The conclusion reiterates quinoline's potential as a versatile compound with broad-spectrum applications in treating multiple health conditions.


![QUINOLINE
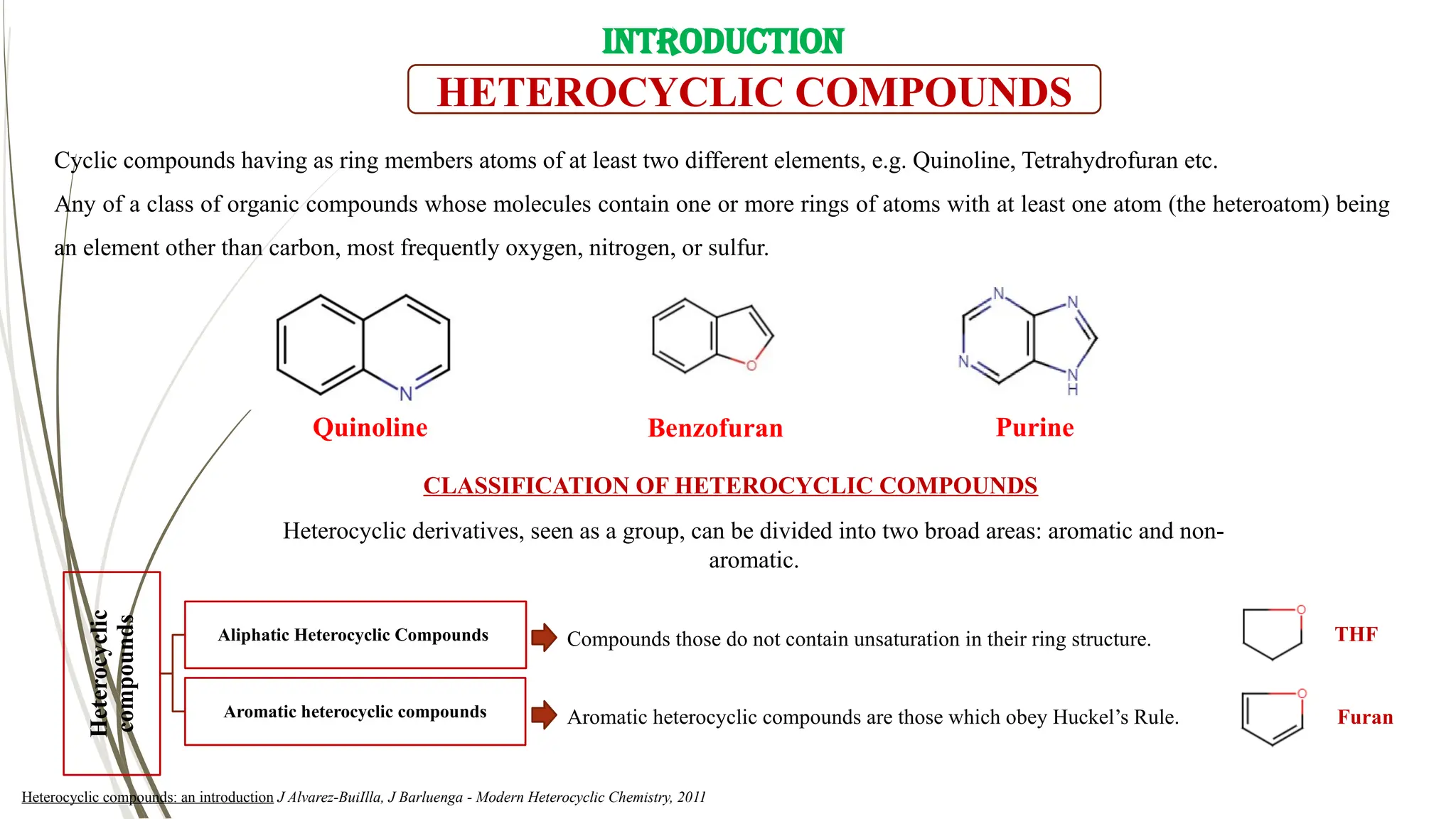
Quinoline or 1-aza-naphthalene or benzo[b]pyridine is a planner hetero-aromatic compound
in which 10π electrons move throughout the structure and having a molecular formula of C9H7N.
Quinoline reported as an important building block in the field of medicinal chemistry.
QUINOLINE
Properties of Quinoline
Quinoline is colorless liquid and having a peculiar odor.
It is a hygroscopic liquid .
It is soluble in water and completely miscible in ether, alcohol and acetone.
Chemically it gives every reaction of pyridine .
Quinoline readily gives electrophilic substitution reactions of benzene ring.
The Chemistry of Quinolines. RH Manske - Chemical Reviews, 1942 - ACS Publications
Quinoline was first discovered by Friedlieb Rouge in 1834, obtained by the distillation of coal tar.
However, its structure, comprised of a rigid heterocyclic core of benzene ortho-fused with a pyridine
ring , was only unveiled in 1871 as Dewar observed the chemical similarity between pyridine and
quinoline](https://image.slidesharecdn.com/quinolines-240827063434-eb2fa46c/75/synthesis-of-quinoline-derivatives-and-its-applications-4-2048.jpg)