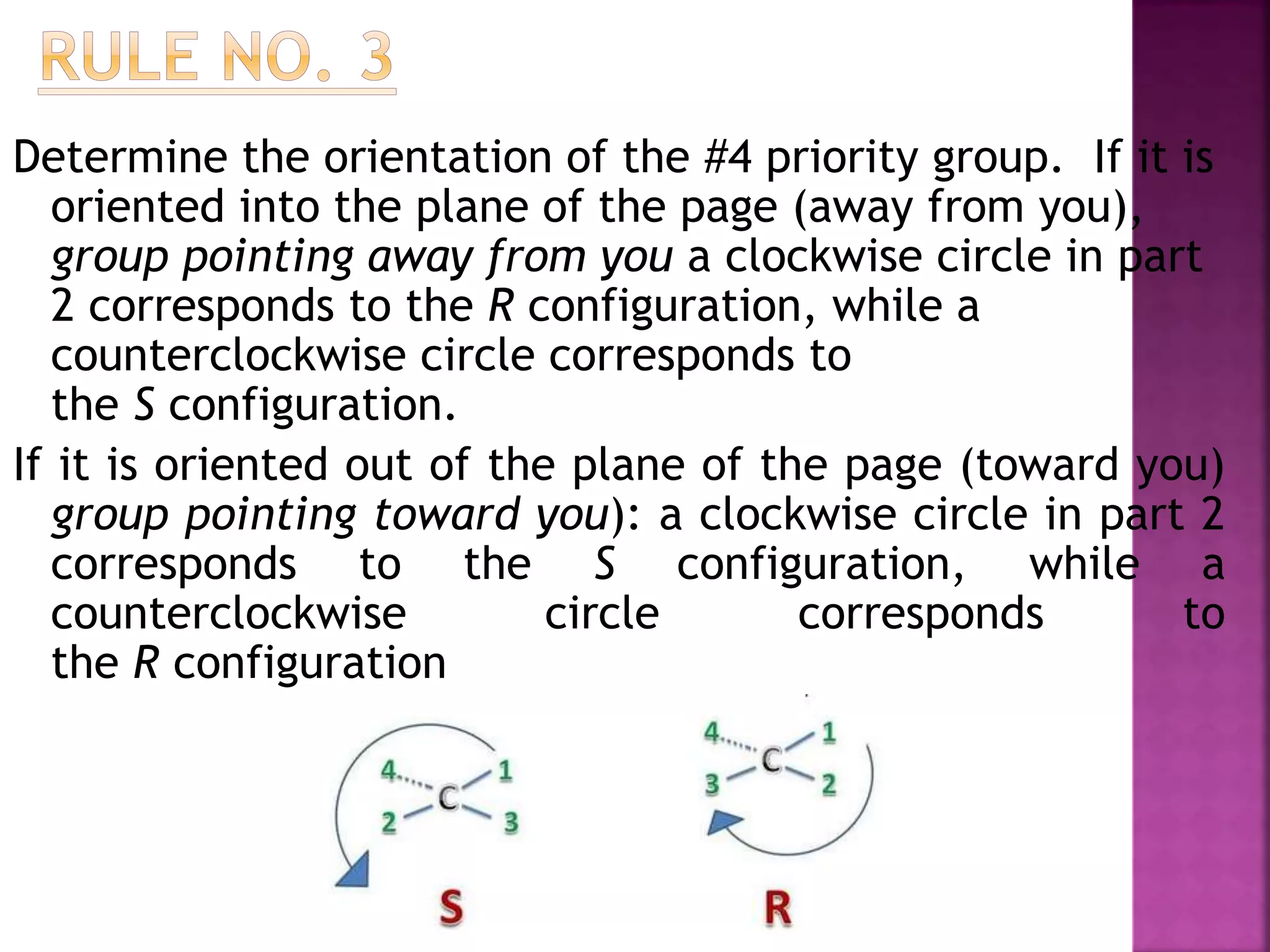
The document outlines rules for assigning R and S configurations using the Cahn-Ingold-Prelog priority system. It discusses 3 steps: 1) Assigning priority numbers 1-4 to substituents based on atomic number, 2) Tracing a circle from the #1 to #2 to #3 substituents, and 3) Determining if the #4 substituent is oriented into or out of the page plane to assign it as R or S. Examples are provided to demonstrate applying the 3 steps to assign stereochemical configuration unambiguously.