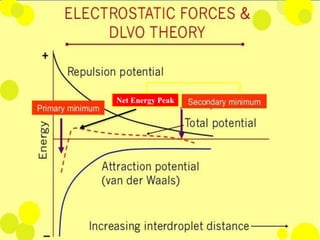
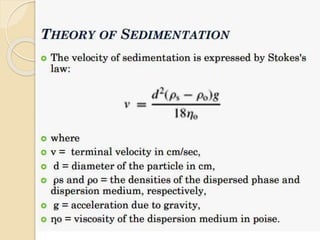
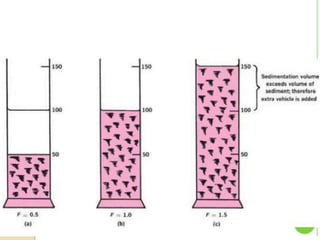
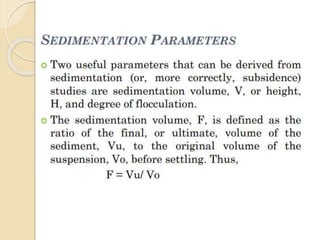
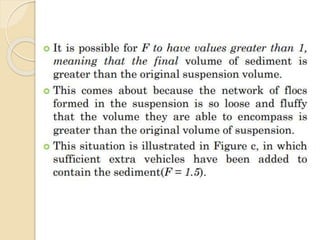
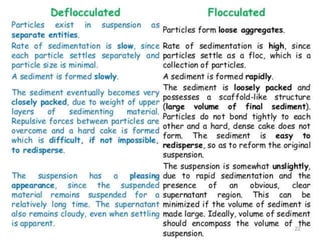
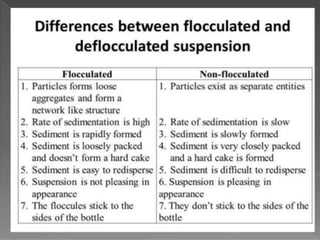
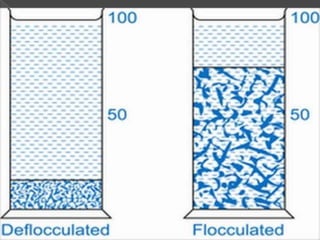
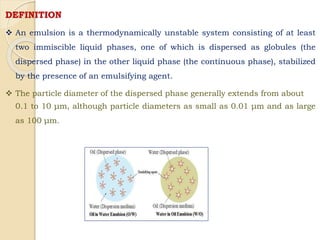
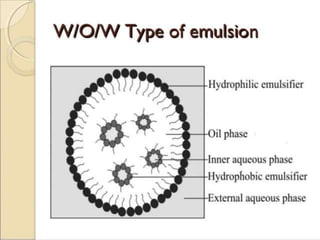
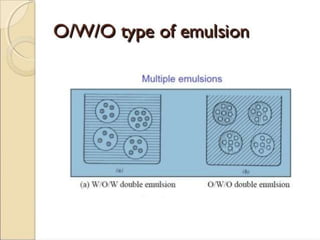
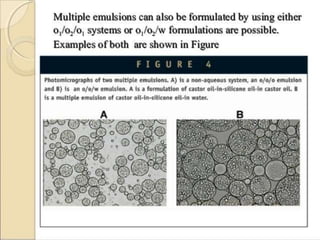
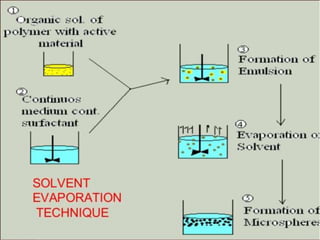
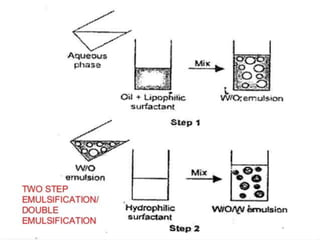
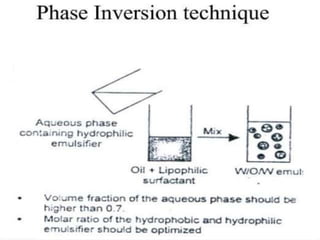
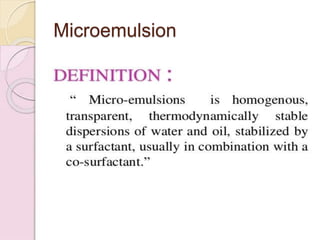
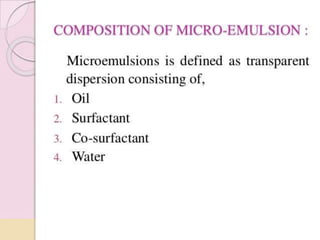
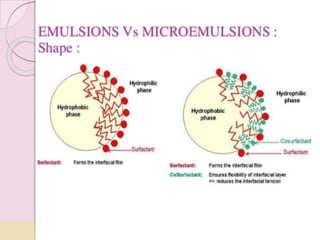
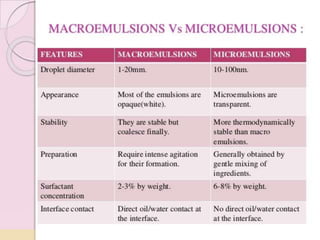
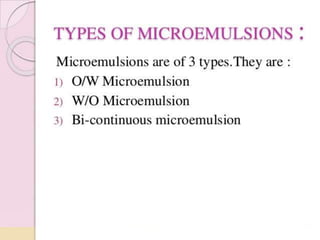
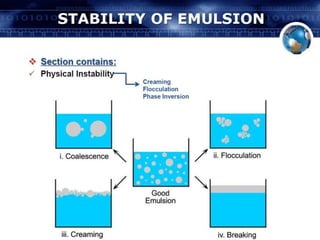
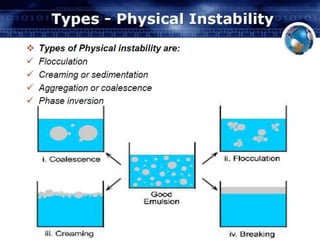
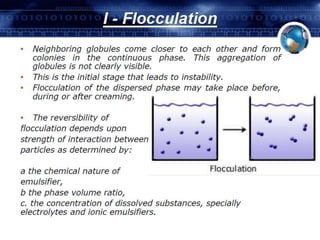
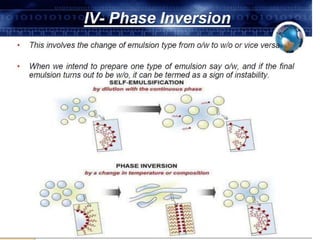
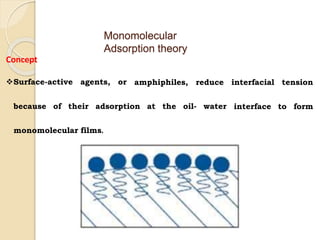
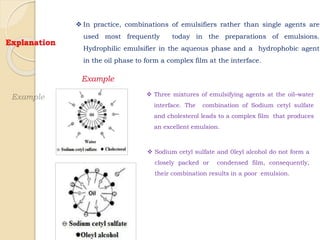
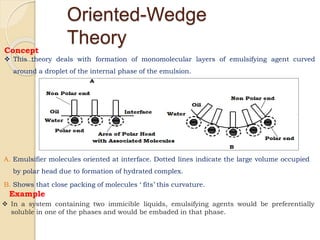
Coarse dispersions are heterogeneous systems where the dispersed particles are larger than 1000 nm. They are characterized by relatively fast sedimentation. The dispersed phase may be easily separated from the continuous phase by filtration. A pharmaceutical suspension is a coarse dispersion where the internal phase is uniformly dispersed throughout the external phase. The internal phase typically has particle sizes between 0.5-5 microns. Suspensions demonstrate properties like pseudoplasticity and thixotropy which influence stability during manufacture and storage.