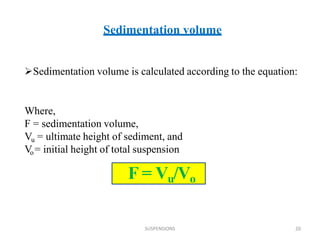
This document discusses pharmaceutical suspensions. A suspension is a coarse dispersion where an insoluble solid drug is dispersed throughout a liquid medium. Suspensions are formulated when drugs are insoluble, to mask bitter taste, increase stability, or allow controlled drug release. Common types include oral, topical, and injectable suspensions. Key factors in suspension quality include particle size, stability, redispersibility, and uniform dosing. Evaluation methods examine sedimentation, viscosity, zeta potential, particle size distribution, and response to freeze-thaw cycles. Proper formulation involves selection of suspending agents, preservatives, buffers, and packaging in containers allowing for redispersion.