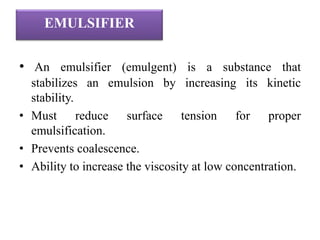
This document discusses semi-solid dosage forms, including their definition, advantages, disadvantages, and ideal properties. It describes various types of semi-solid dosage forms such as ointments, pastes, creams, gels, and foams. It covers the ingredients used to prepare these forms, such as bases, preservatives, emulsifiers, and antioxidants. It also discusses factors that influence drug penetration through the skin from these dosage forms and different preparation methods for ointments specifically, including incorporation, fusion, and chemical reaction methods.