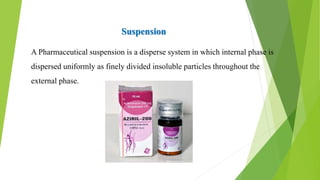
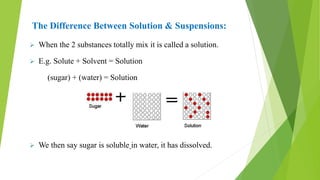
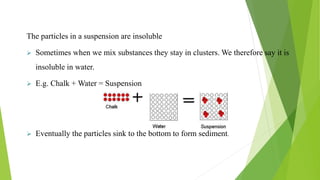
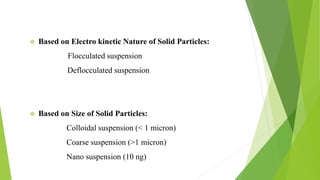
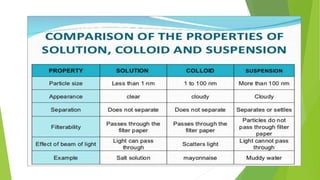
A pharmaceutical suspension is a dispersed system where insoluble particles are uniformly distributed throughout a liquid medium. Key aspects of suspensions include the particle size, shape, interactions, and use of suspending agents to decrease aggregation. Suspensions are used to deliver insoluble drugs and control drug release while masking unpleasant tastes. Quality is ensured through tests of sedimentation, rheology, stability, and redispersibility.