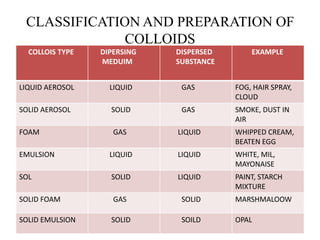
1) Colloids are heterogeneous mixtures where one substance is dispersed evenly throughout another. They can exist as solid aerosols, foams, emulsions, sols, or solid foams.
2) Colloids exhibit unique optical properties like the Tyndall effect where light is scattered by colloidal particles. They also display random Brownian motion from bombardment by the dispersion medium.
3) Colloids can be classified and prepared through various processes like condensation, dispersion, or using emulsifying agents. Their stability is important and they may undergo destabilization through phenomena like sedimentation or flocculation.