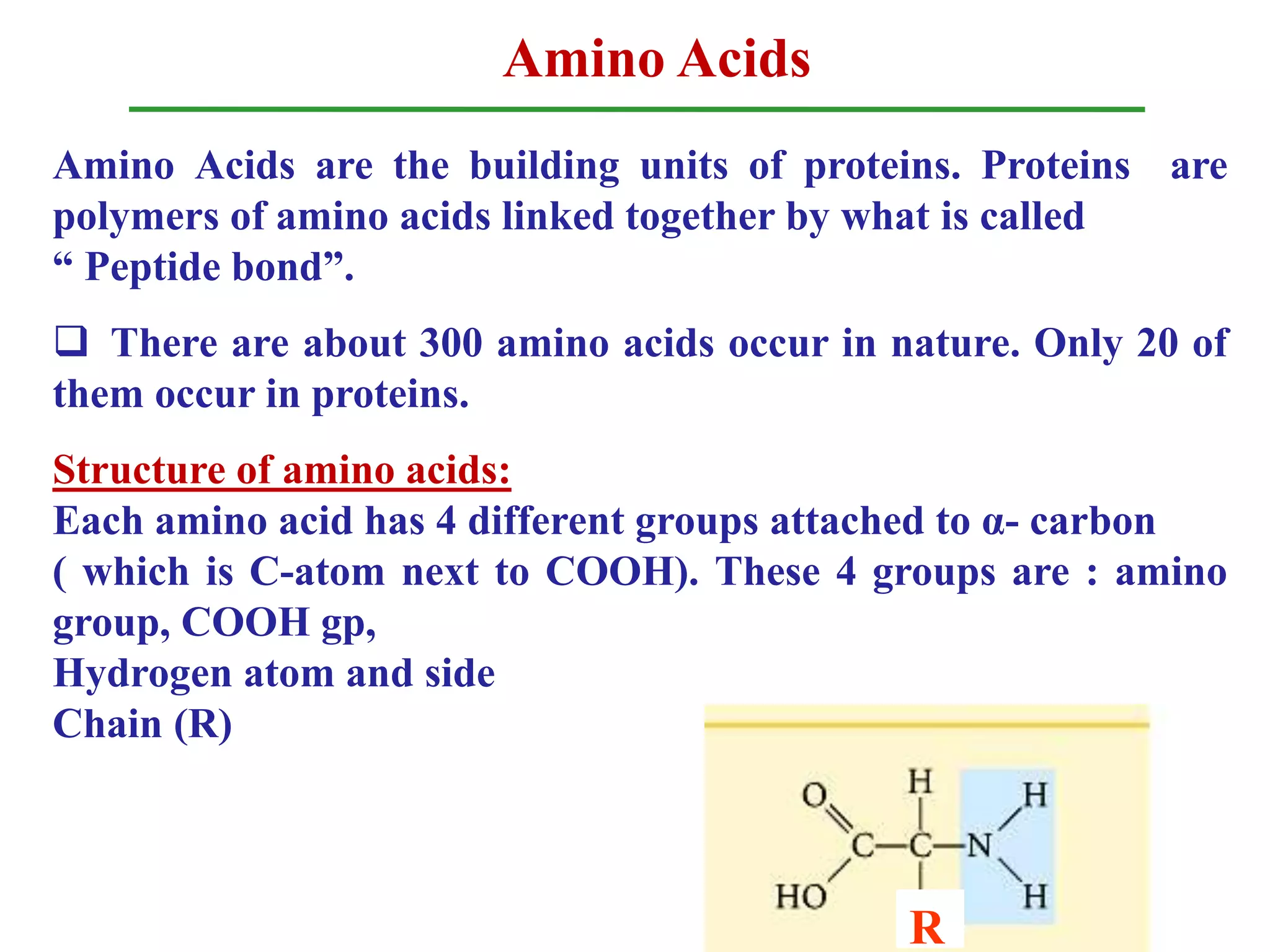
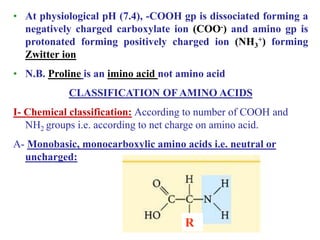
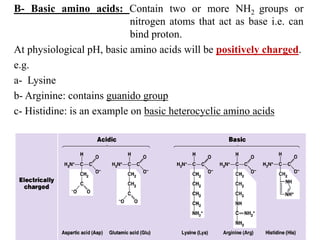
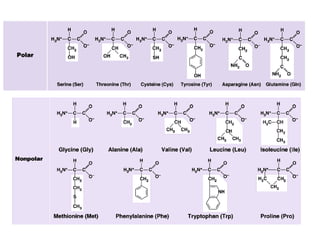
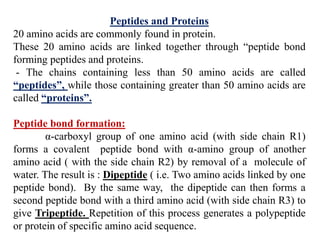
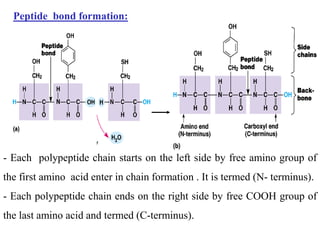
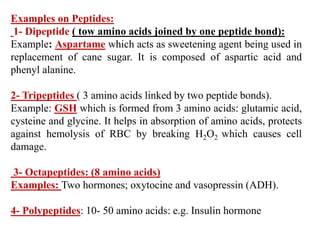
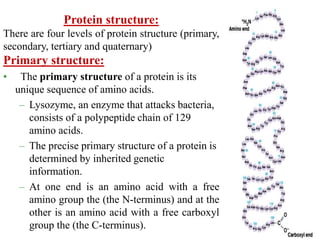
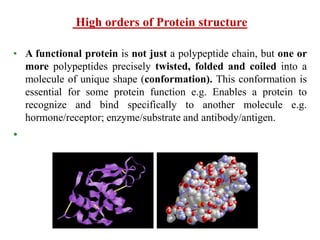
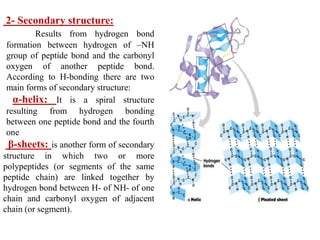
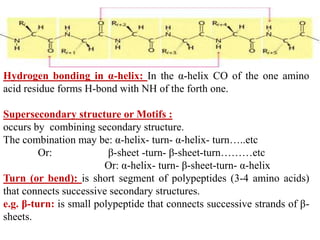
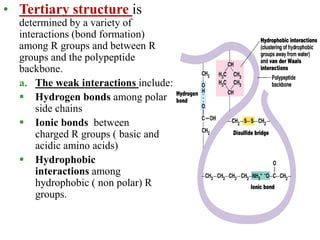
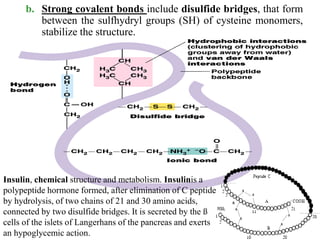
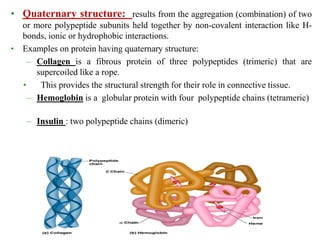
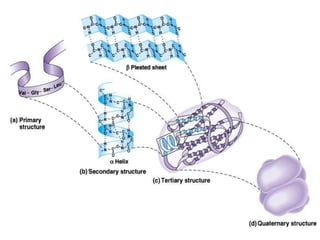
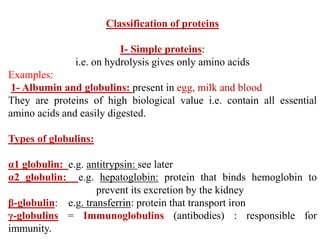
Amino acids are the building blocks of proteins, with around 300 occurring in nature but only 20 being protein constituents. They can be classified based on chemical structure, polarity, nutrition, and metabolism, with distinctions such as essential, semi-essential, and non-essential amino acids. Proteins, formed from amino acids via peptide bonds, exhibit four structural levels (primary, secondary, tertiary, quaternary), with functions influenced by their specific sequences and conformations.