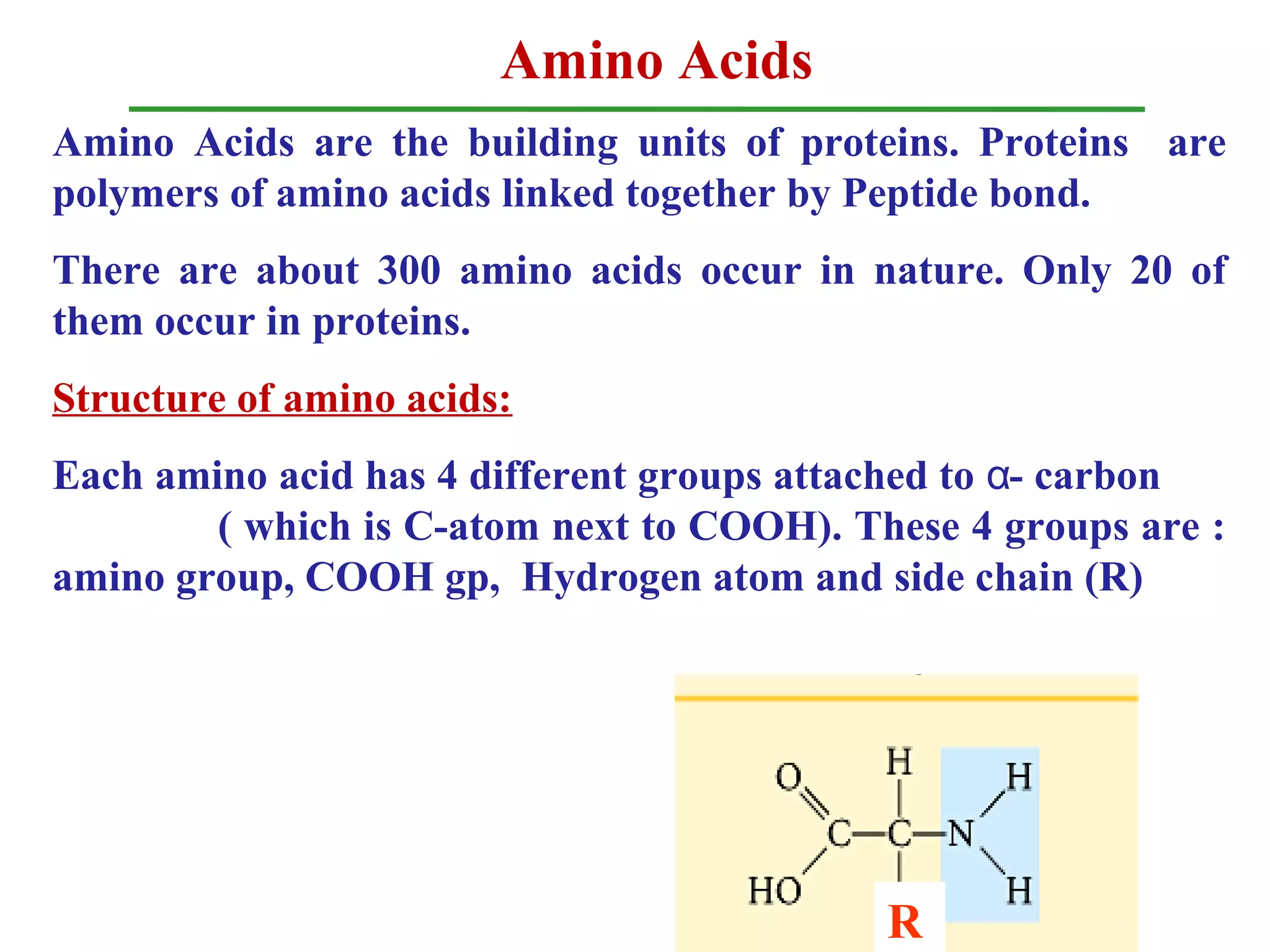
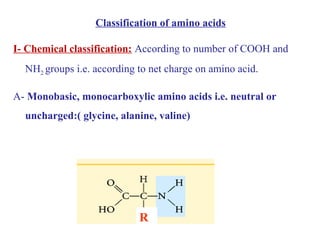
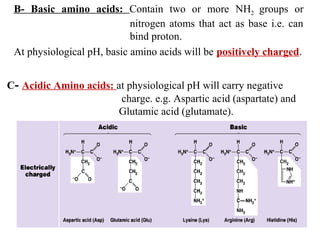
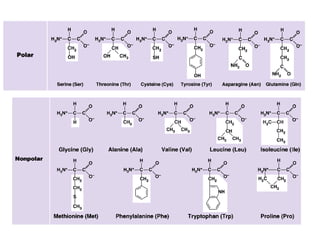
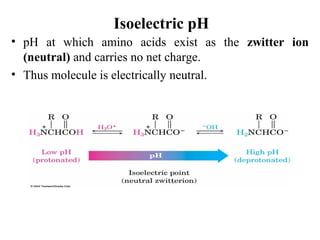
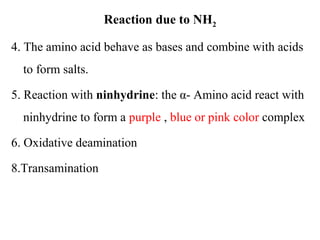
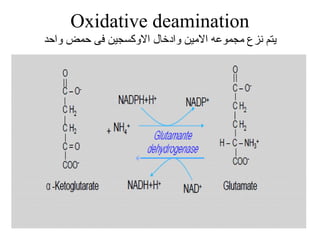
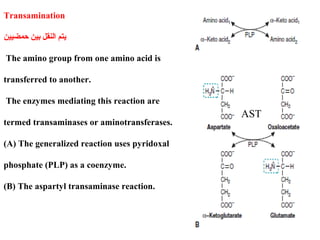
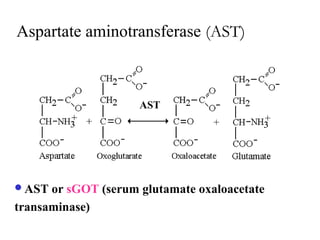
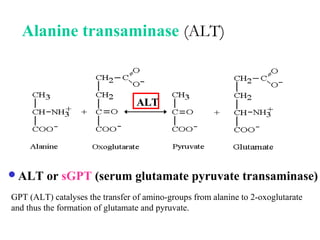
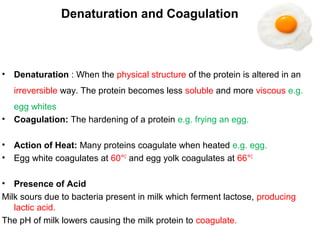
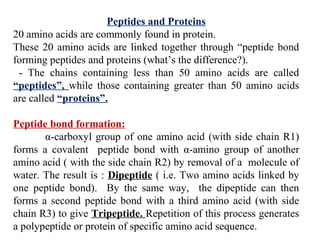
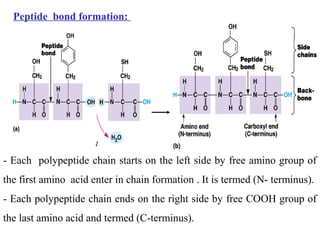
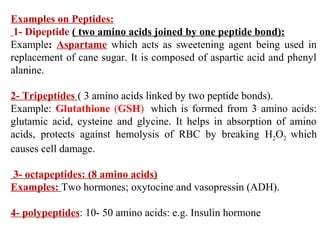
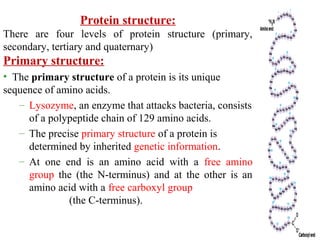
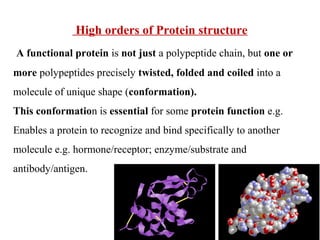
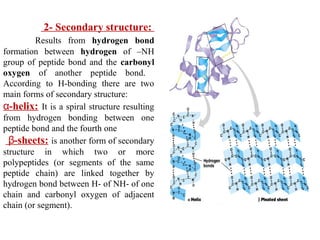
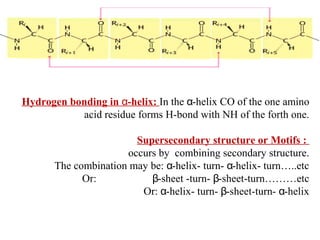
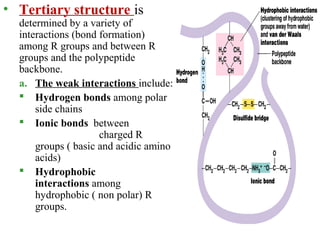
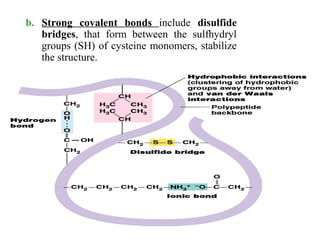
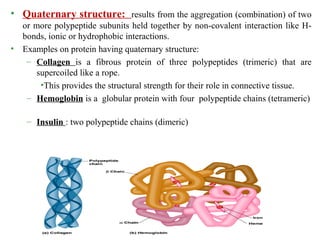
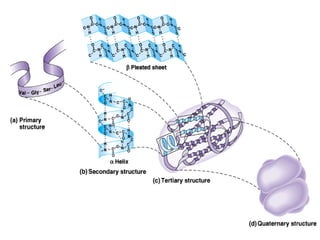
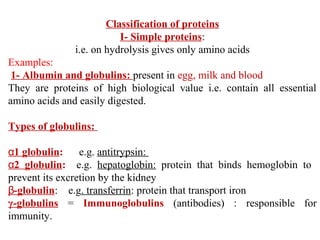
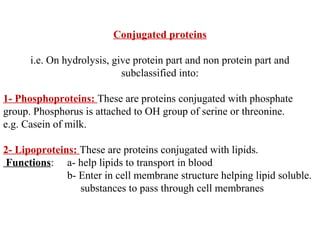
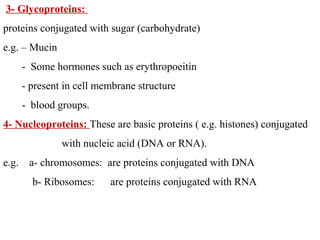
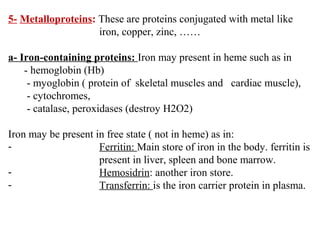
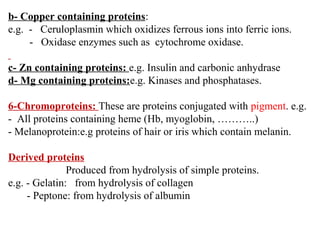
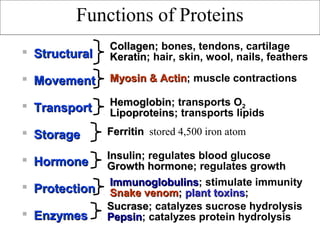
Amino acids are the building blocks of proteins. There are 20 common amino acids that make up proteins through peptide bonds. Amino acids have four groups attached to the alpha carbon including an amino group, carboxyl group, hydrogen, and a variable side chain. Amino acids can be classified based on their structure and properties. Proteins have four levels of structure - primary, secondary, tertiary, and quaternary - which determine their shape and function.