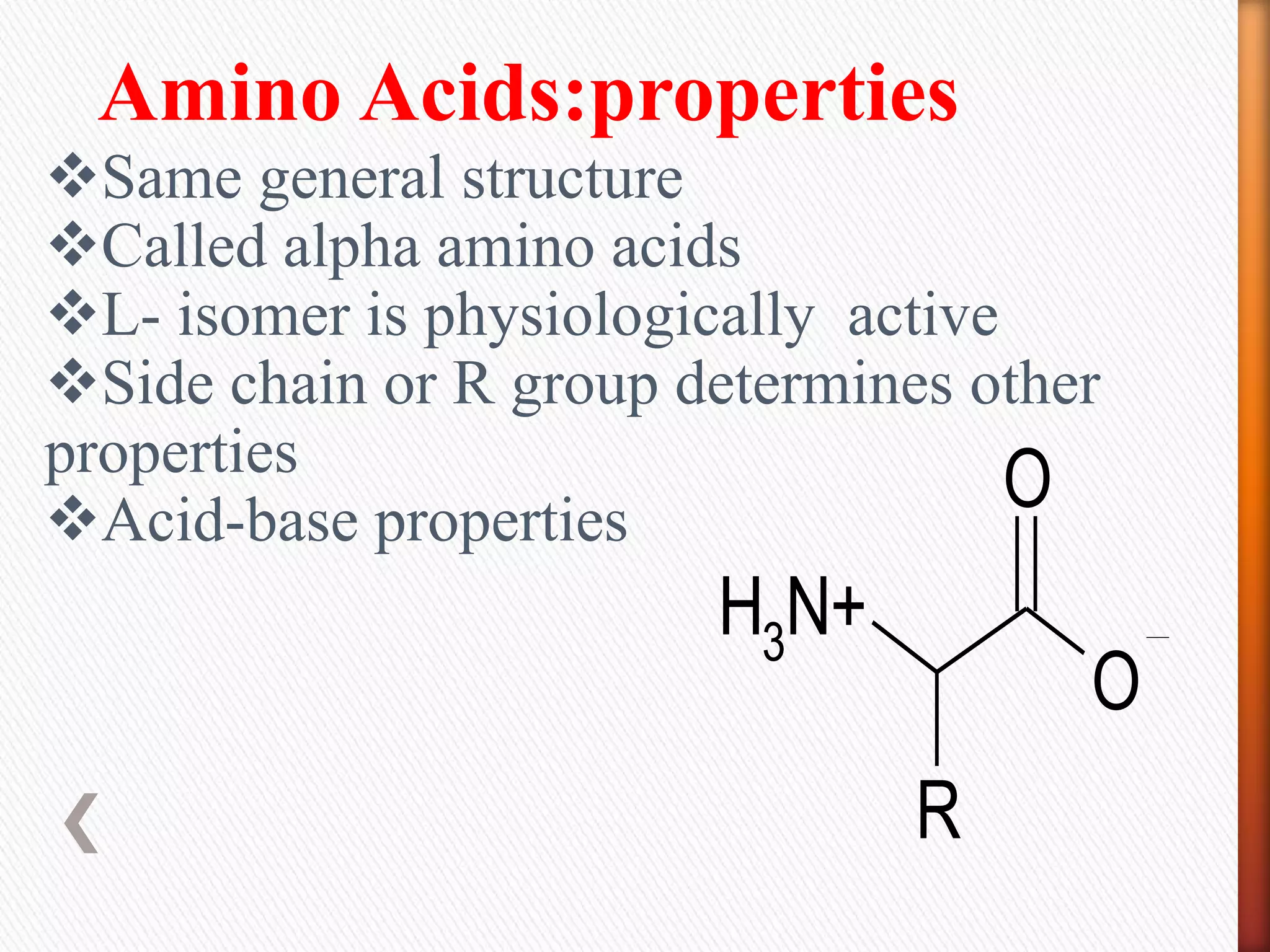
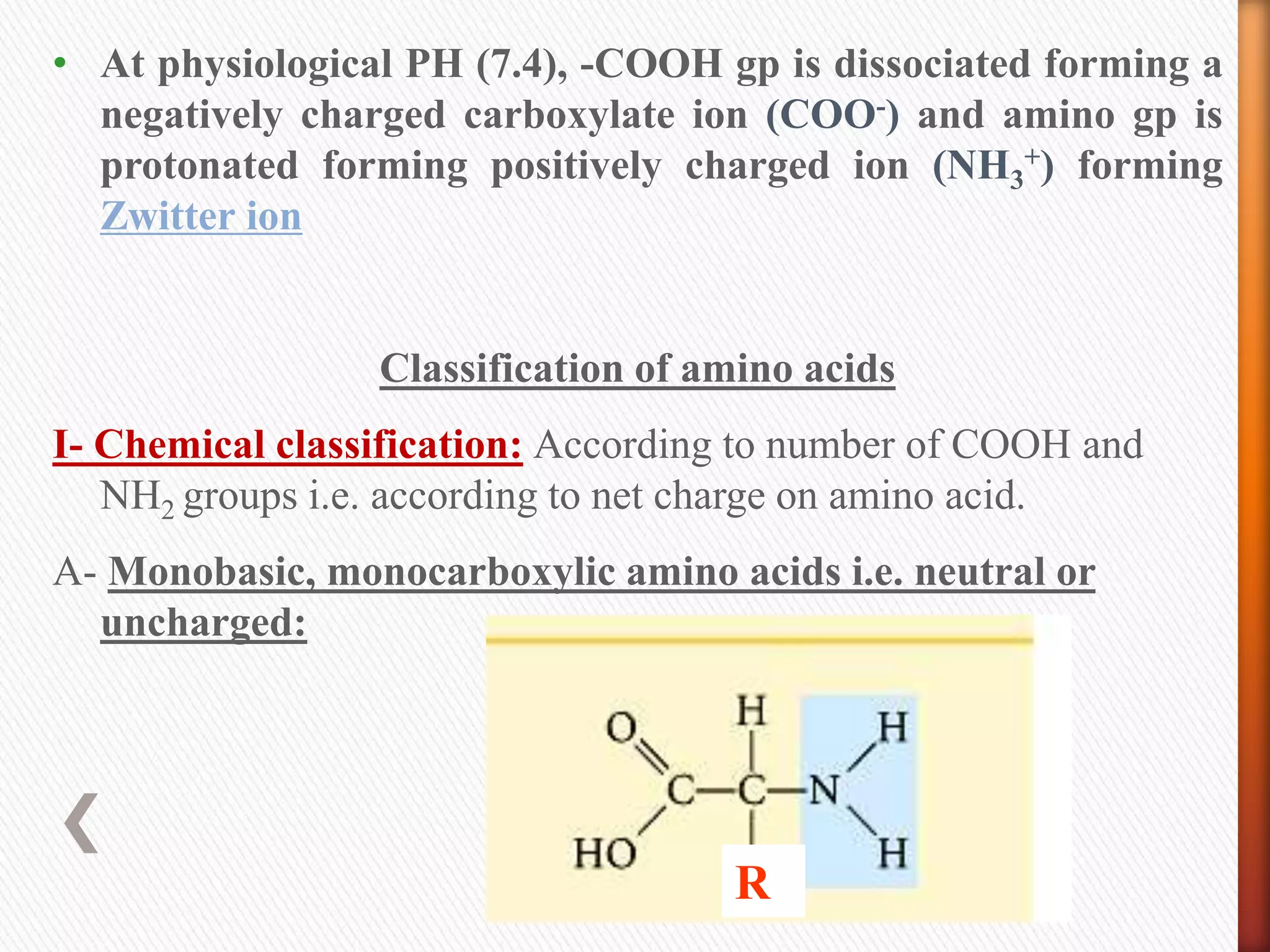
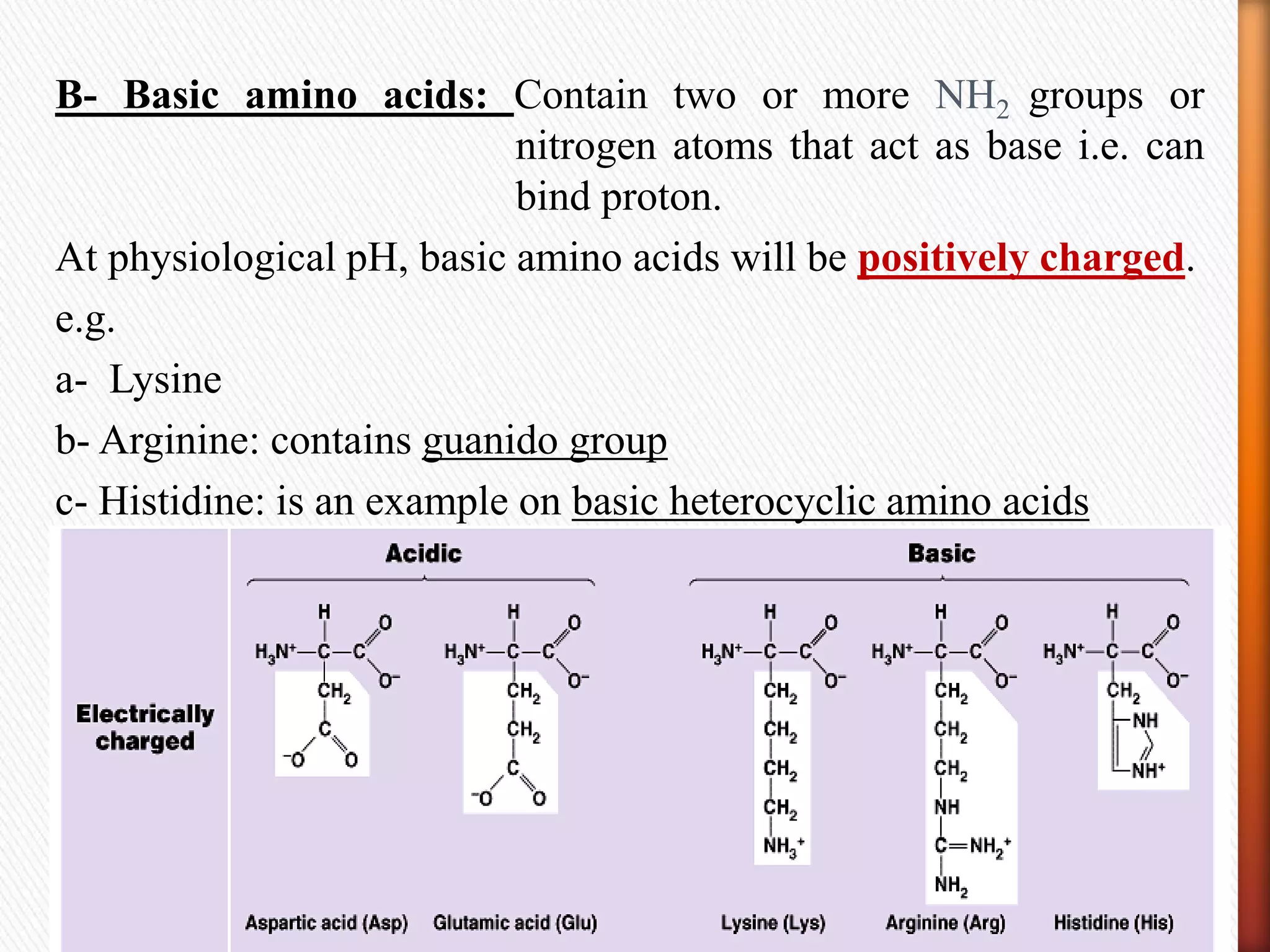
1) Amino acids are the building blocks of proteins and are linked together by peptide bonds to form polypeptides or proteins.
2) There are 20 common amino acids that occur in proteins. Amino acids have an amino group, carboxyl group, hydrogen atom, and variable side chain.
3) Proteins have a primary structure defined by the sequence of amino acids, and higher order structures including secondary structures like alpha helices and beta sheets formed by hydrogen bonding between amino acids.