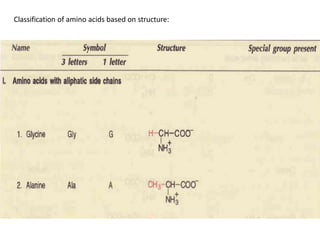
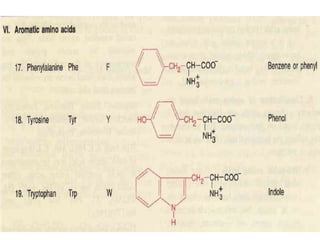
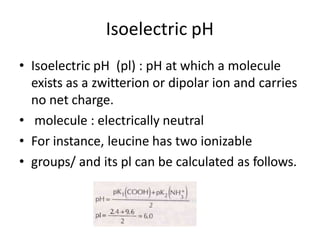
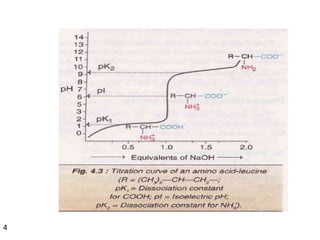
Proteins are composed of chains of amino acids and perform essential functions in the body. There are 20 standard amino acids, some of which are essential and must be obtained through diet. Amino acids link together through peptide bonds to form polypeptide chains or proteins. Proteins have four levels of structure and take on complex shapes that enable their many functions like structure, movement, hormones, and enzymes. A deficiency or excess of proteins can cause health issues.