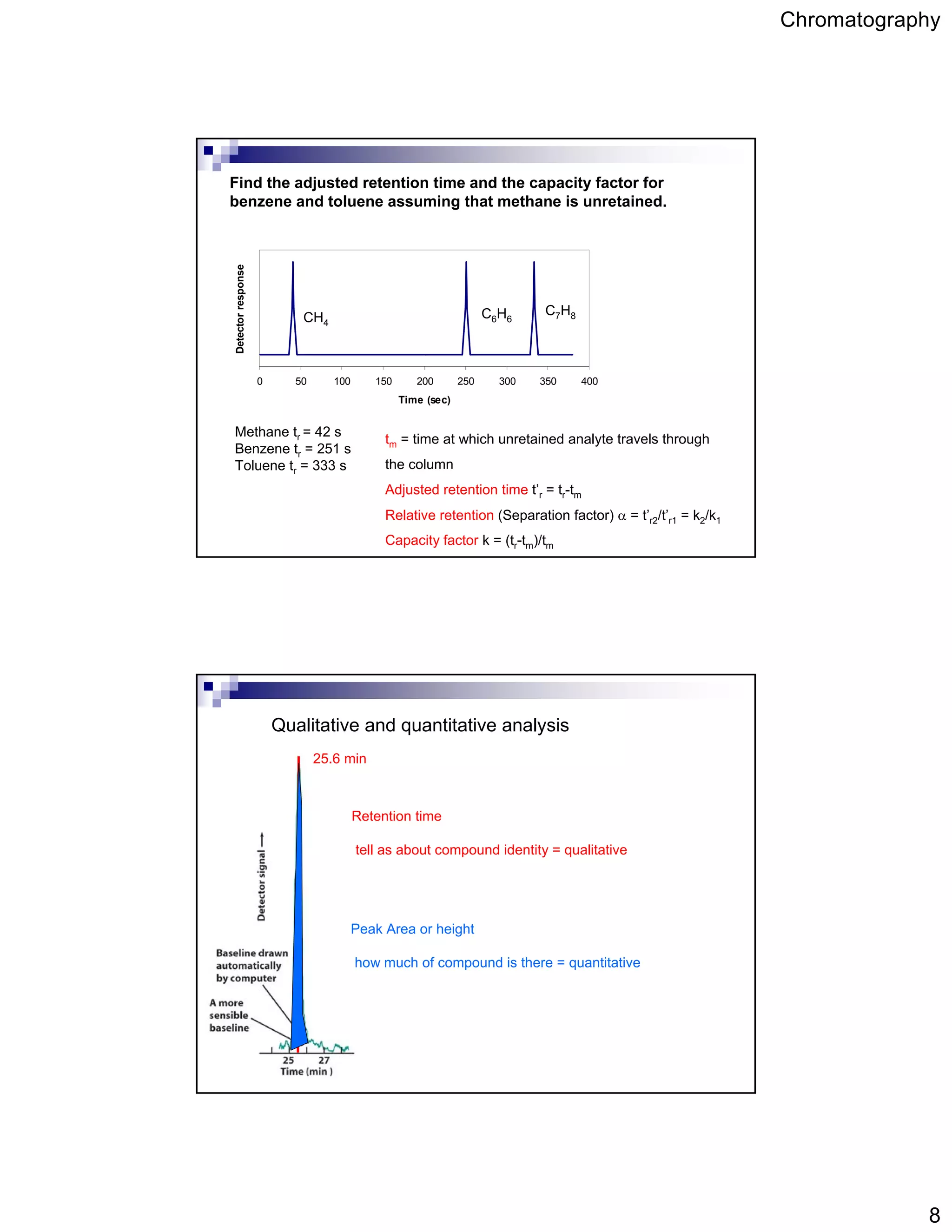
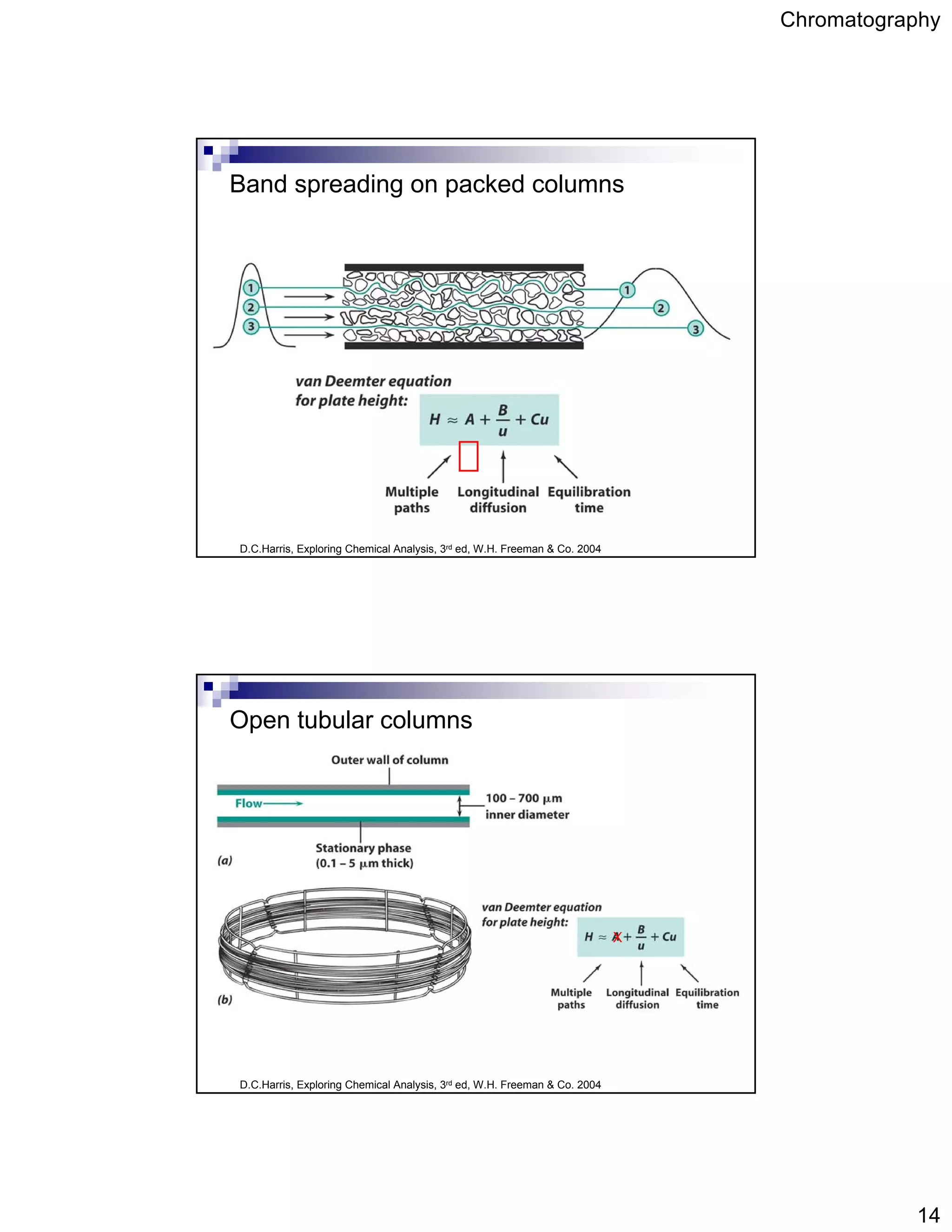
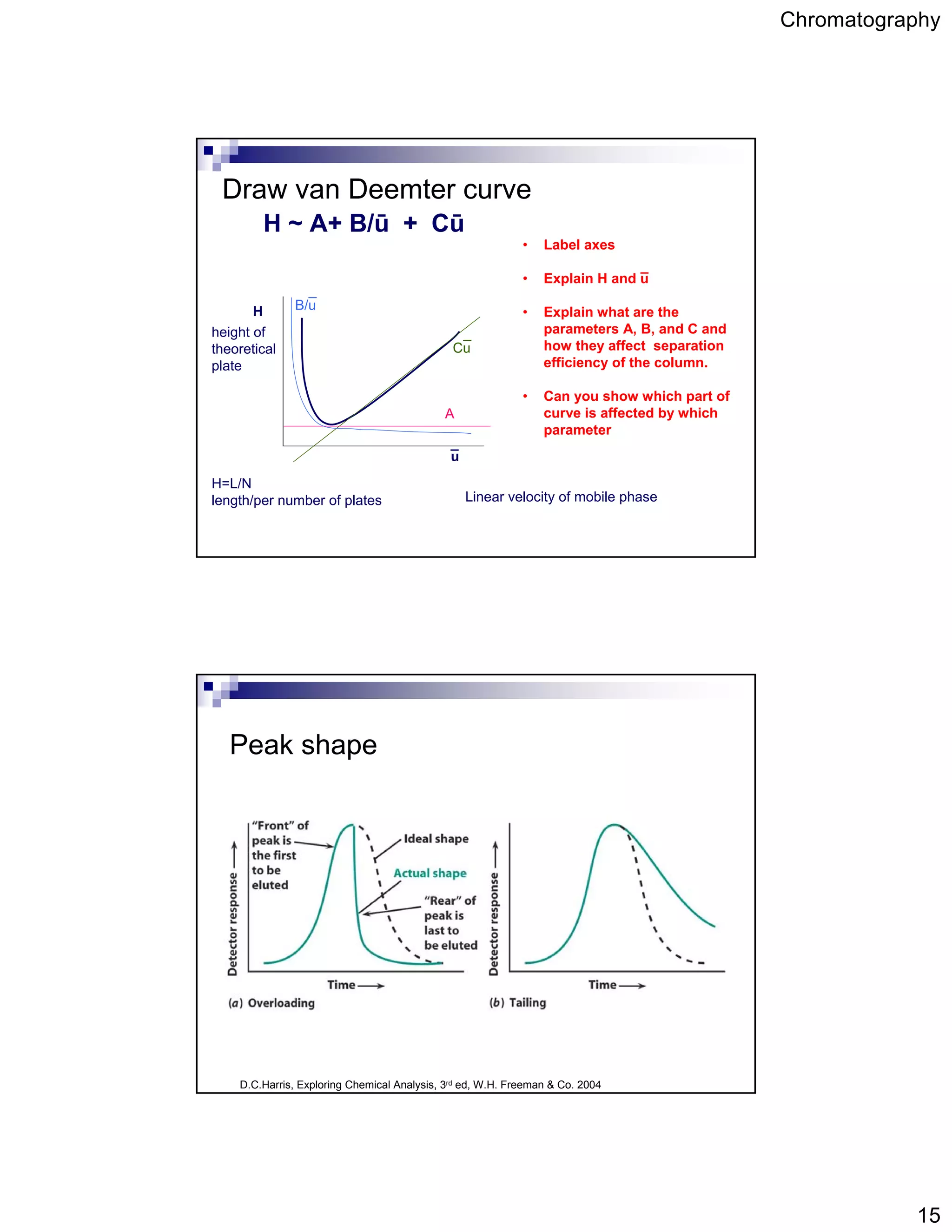
Chromatography is a technique used to separate mixtures by partitioning components between a stationary and mobile phase. It works based on how strongly components interact with and partition between the two phases. Separation is achieved as different components move through the column at different rates and elute at different retention times, creating peaks on a chromatogram. Key aspects of chromatography include the stationary and mobile phases used, retention time, capacity factor, resolution, theoretical plates, and the van Deemter equation which relates column efficiency to flow rate.