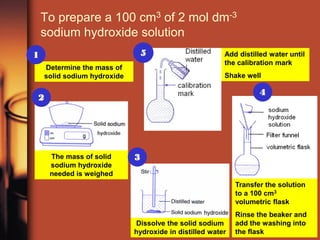
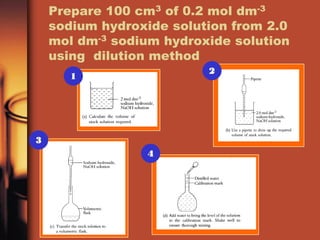
1. Weigh out 8.0g of solid sodium hydroxide and dissolve it in 25cm3 of water in a beaker.
2. Pour the solution into a 100cm3 volumetric flask and rinse the beaker and weighing bottle with water, adding the rinse water to the flask.
3. Add distilled water dropwise until the solution reaches the 100cm3 mark. Shake well to mix.