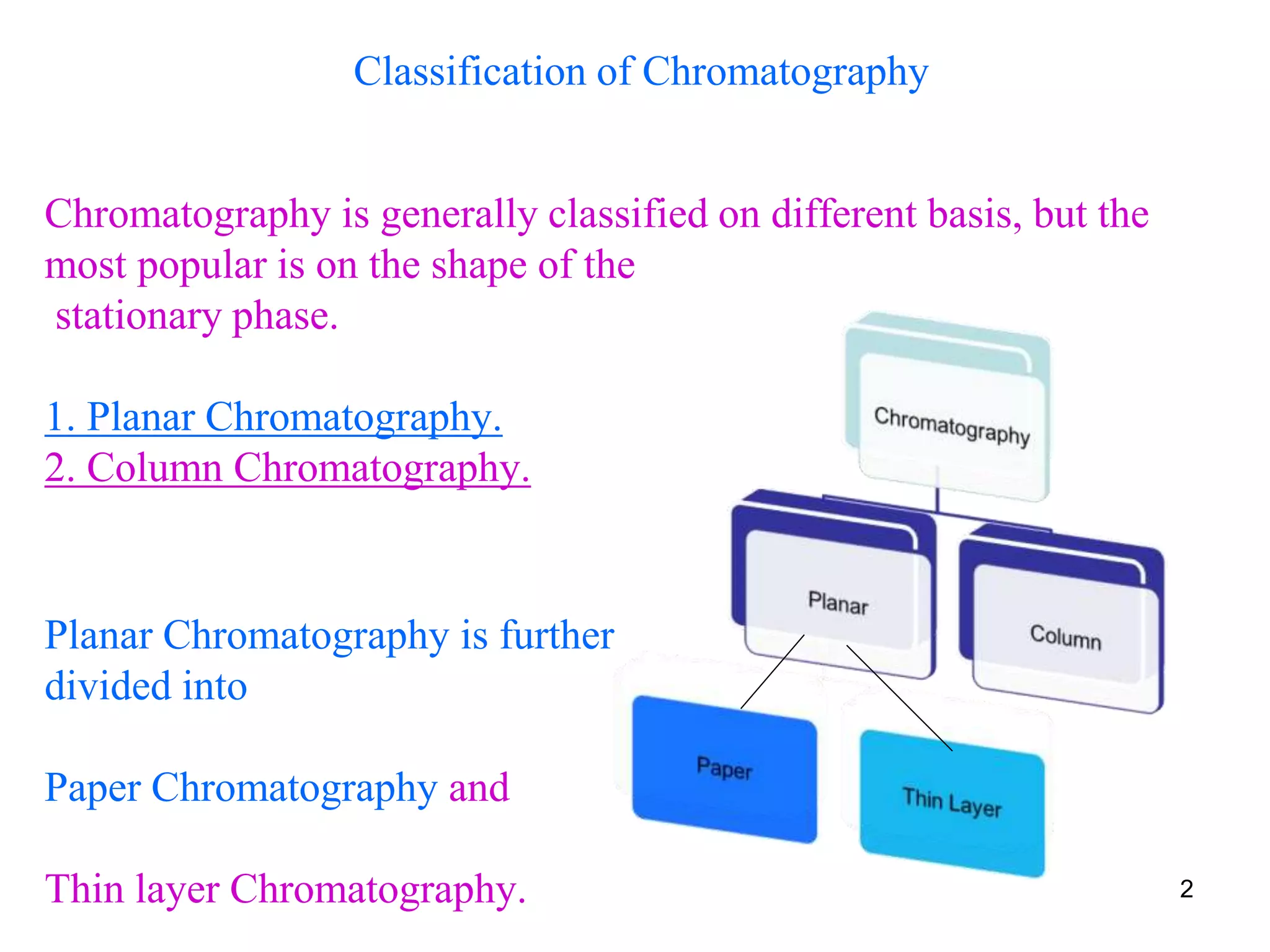
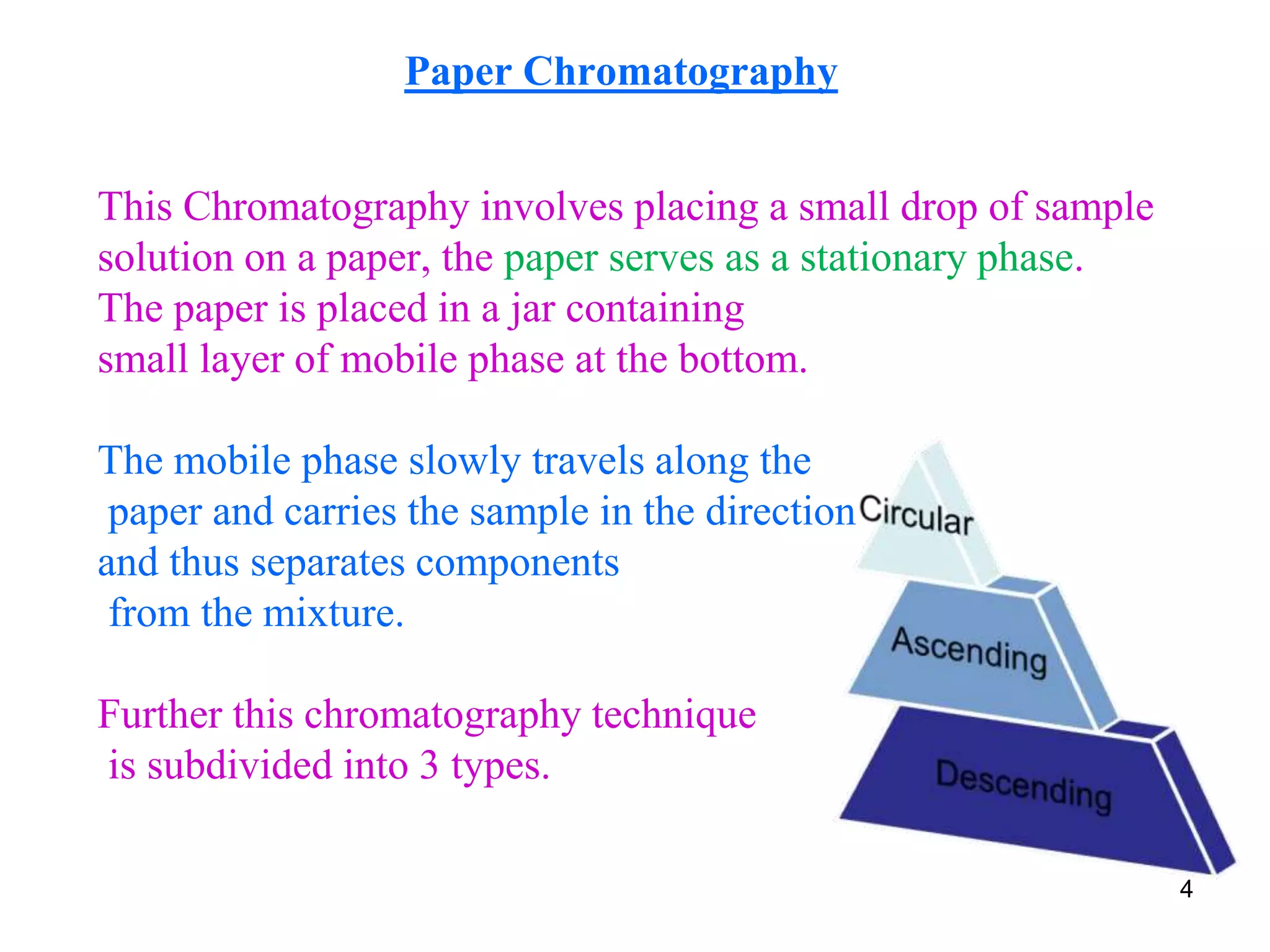
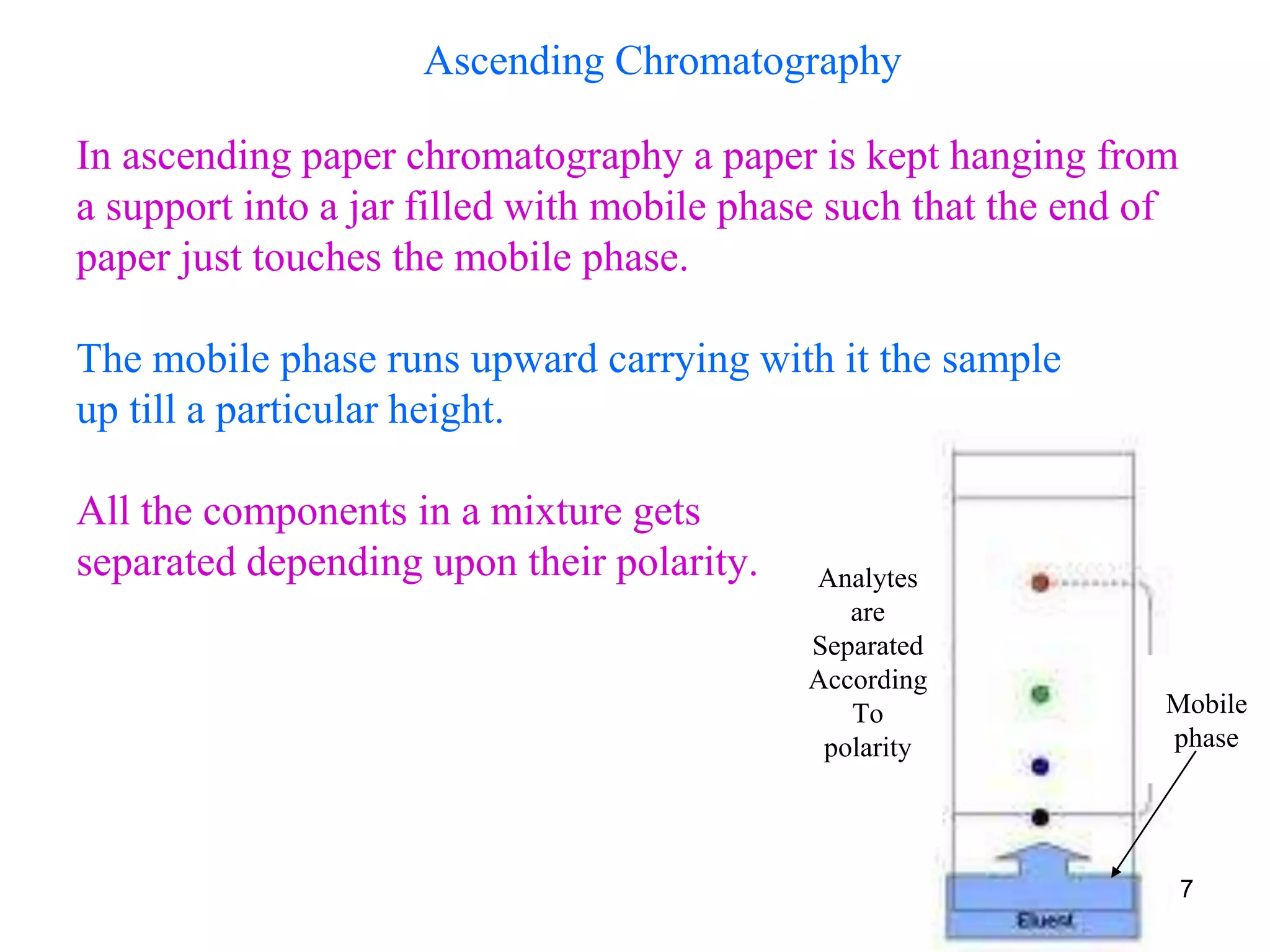
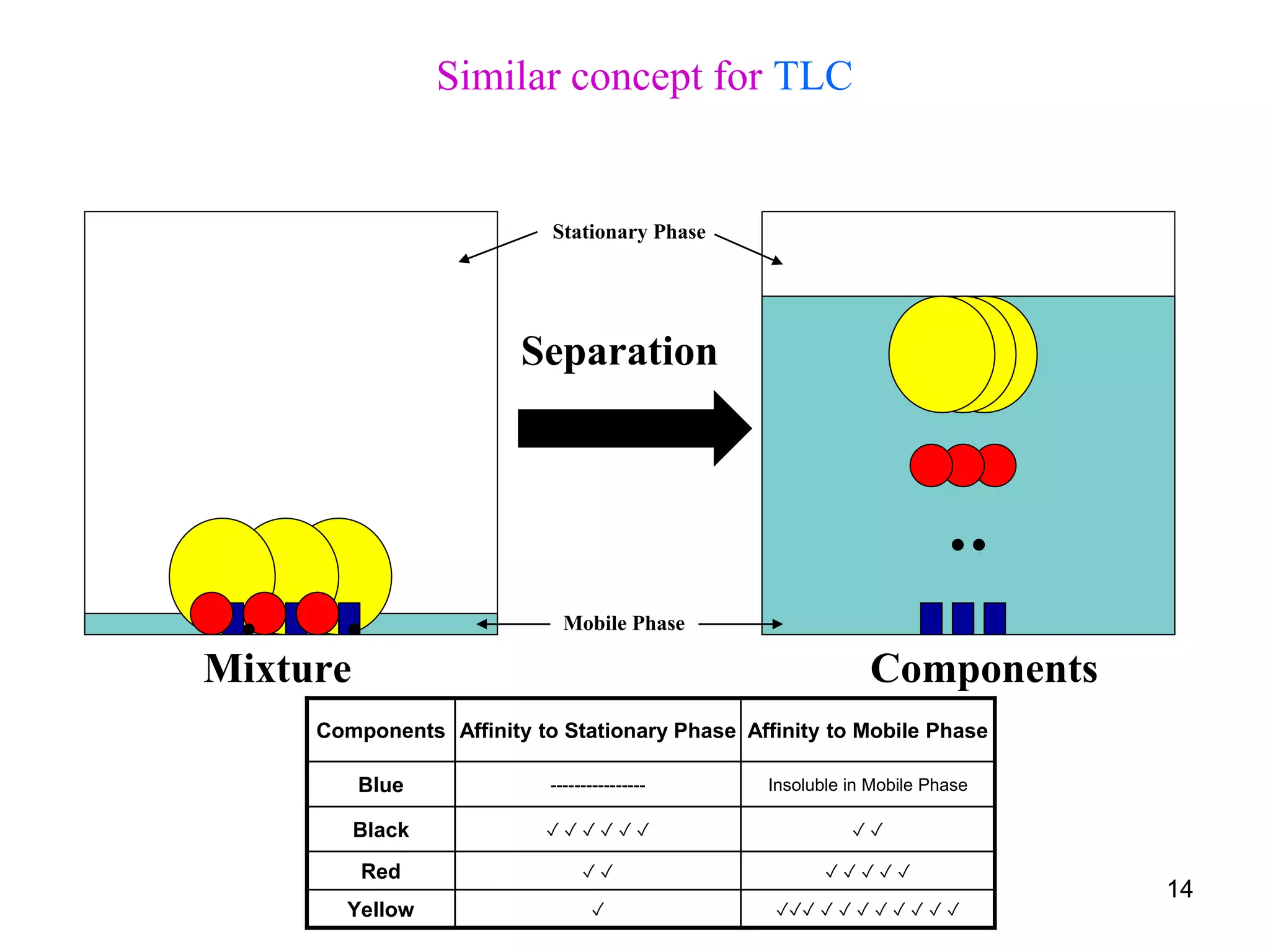
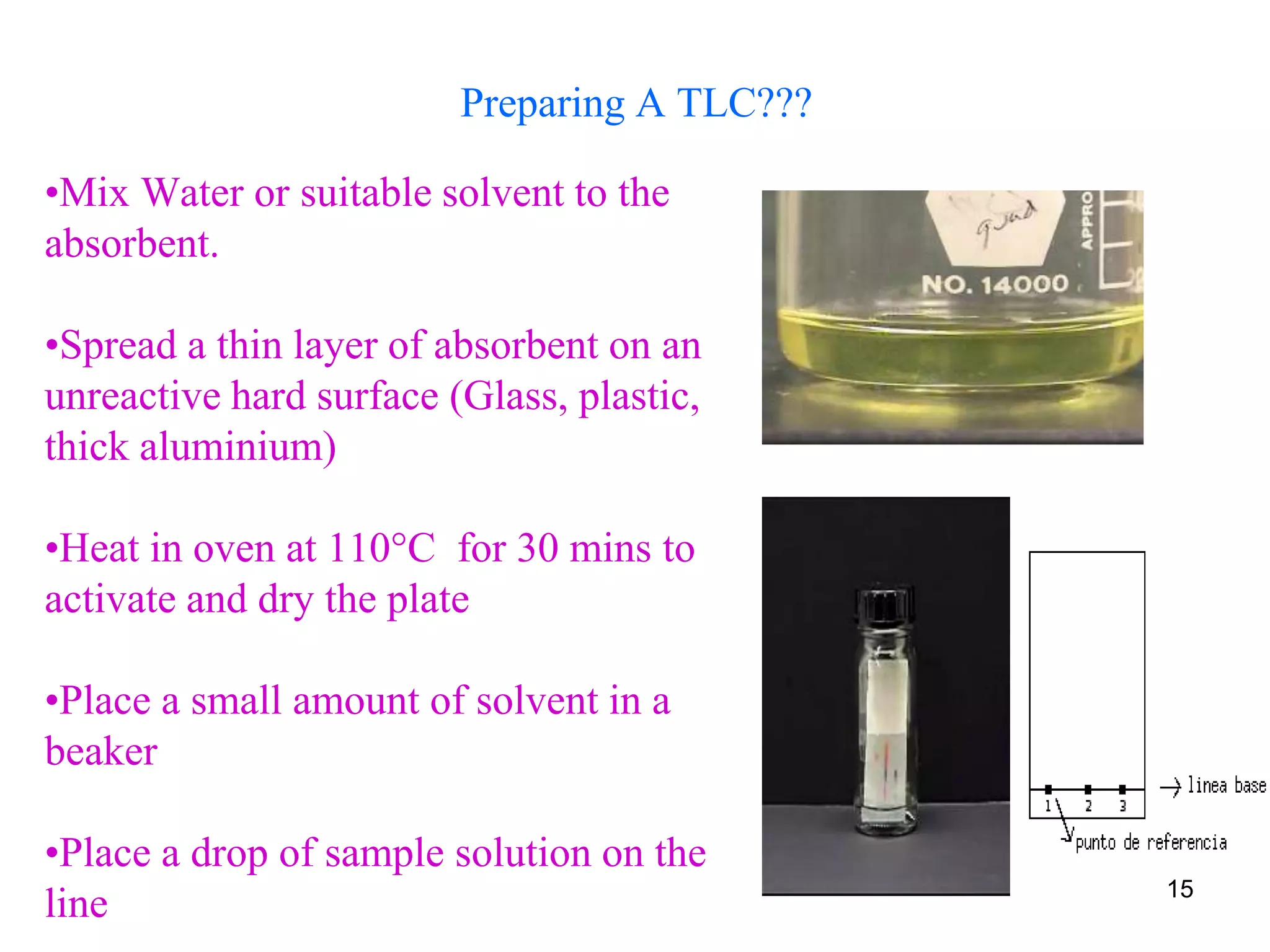
This document discusses different types of chromatography techniques, focusing on paper chromatography and thin layer chromatography (TLC).
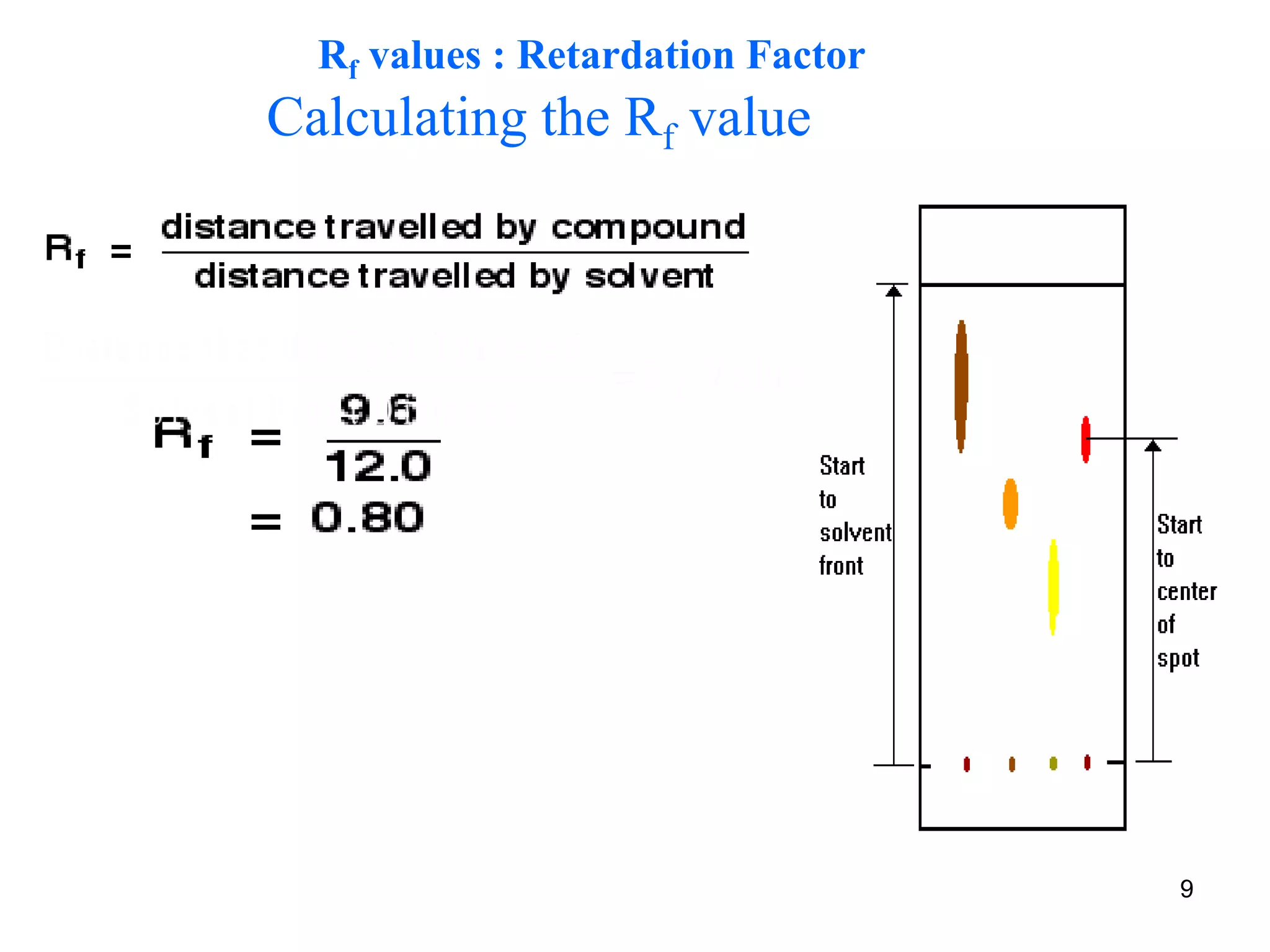
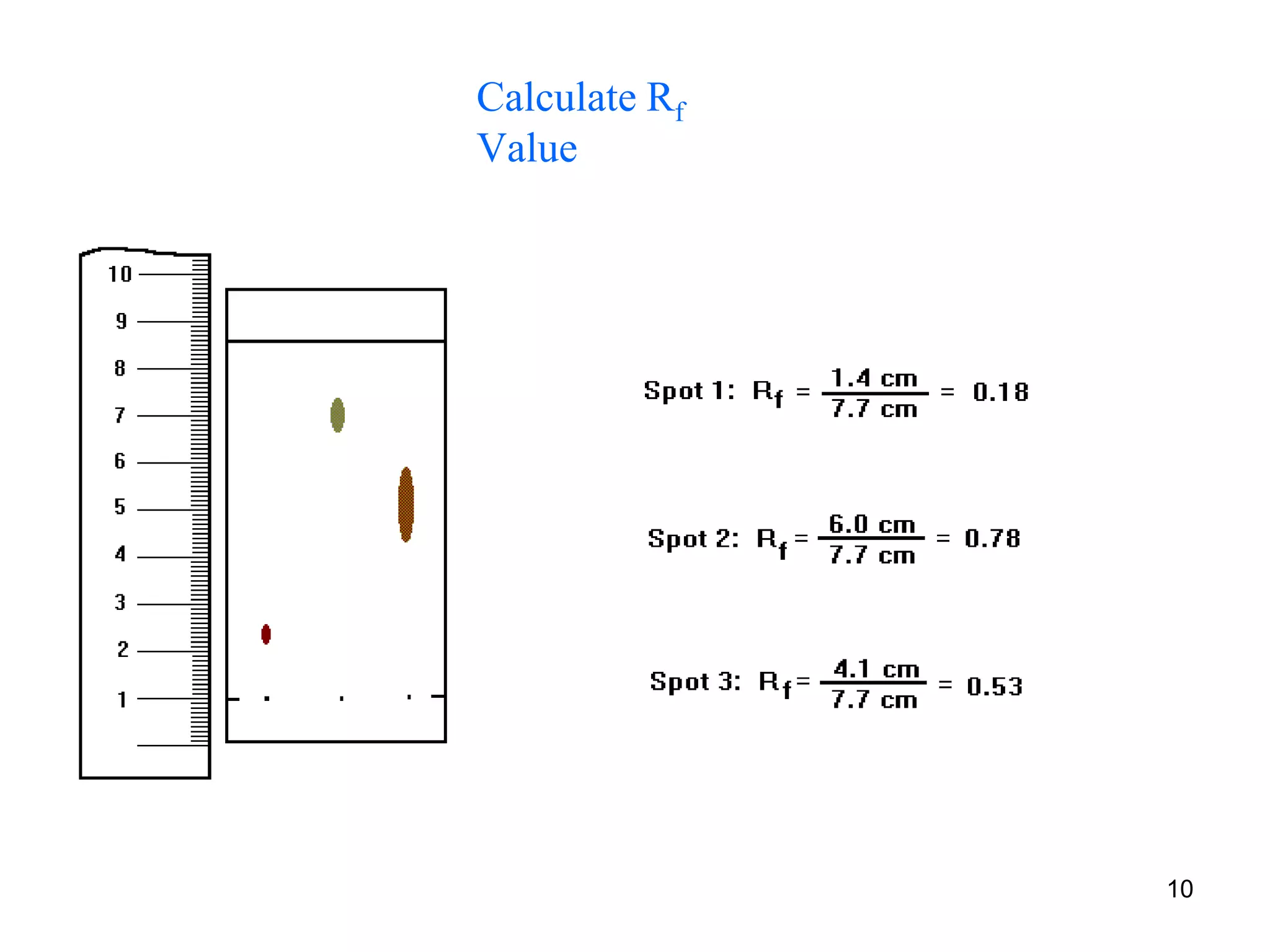
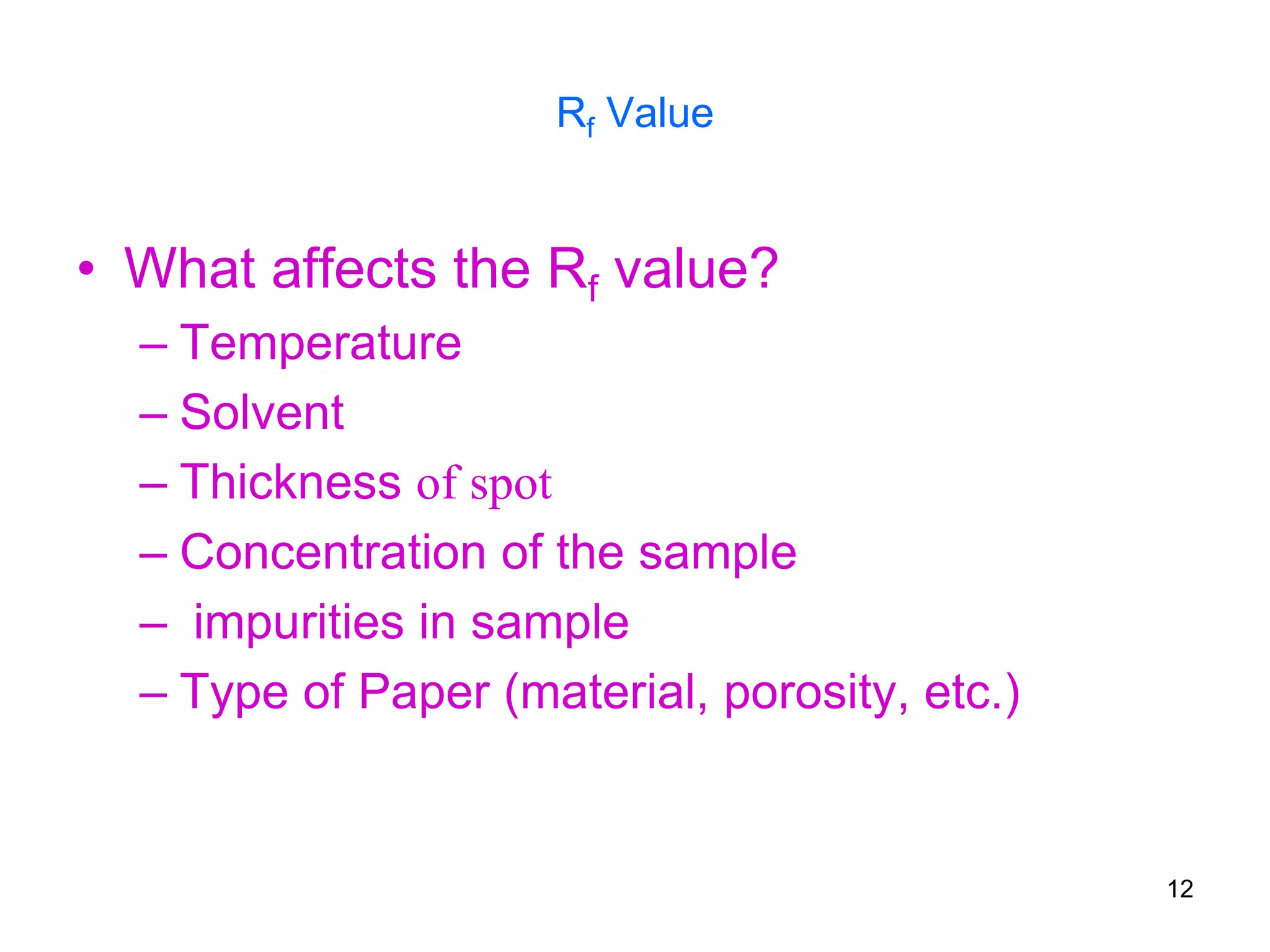
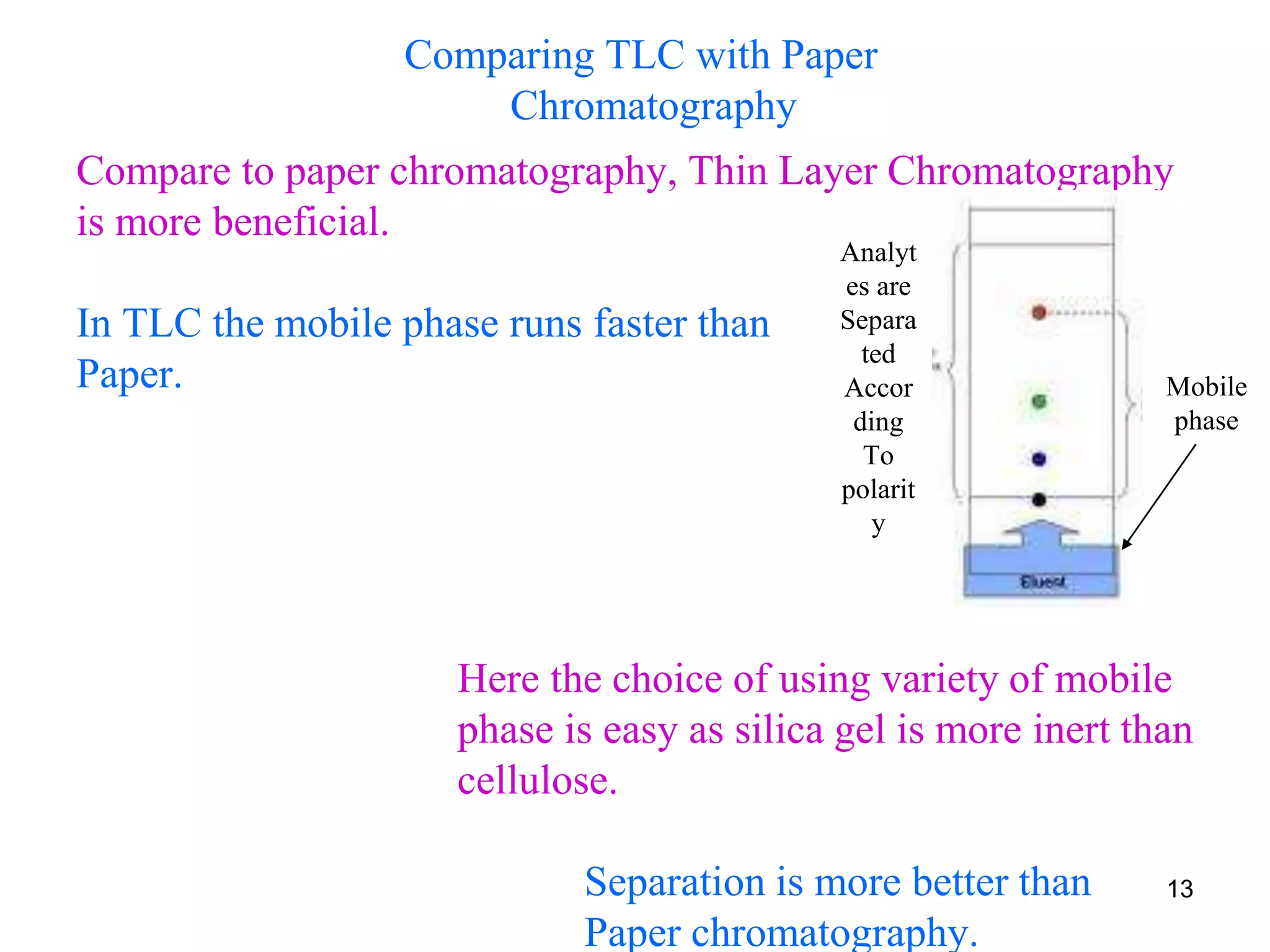
It describes the basic process of paper chromatography, where a small sample is placed on chromatography paper and the paper is placed in a mobile phase, separating the sample components as the mobile phase travels up the paper. Paper chromatography can be ascending, descending, or circular. TLC is similar but uses a silica plate instead of paper and allows for faster separation and more mobile phase options. TLC provides better separation than paper chromatography. The document also discusses calculating Rf values and visualizing results.