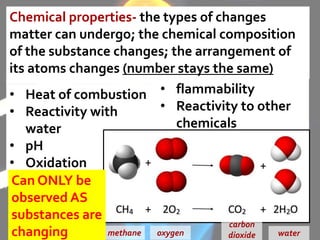
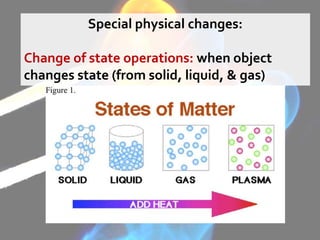
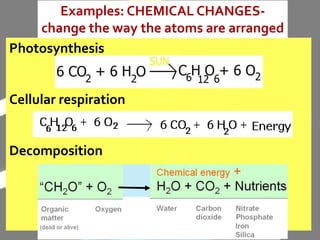
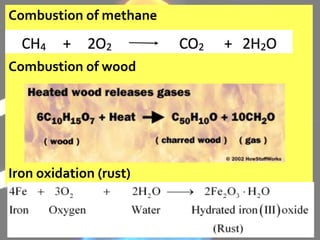
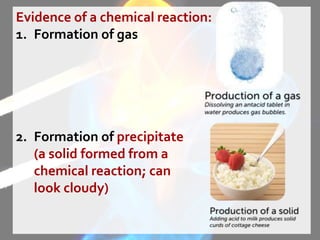
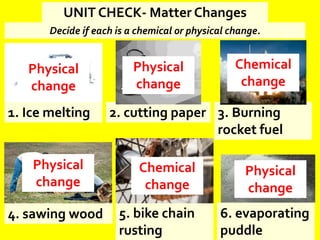
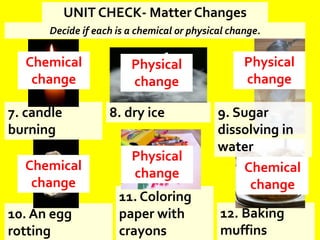
The document discusses the differences between physical and chemical changes in matter. It defines matter as anything that has mass and takes up space. Physical properties can be observed without changing the identity of a substance, while chemical properties involve changes in the chemical composition or atomic structure of a substance. Physical changes alter the physical properties of matter without changing its chemical makeup, while chemical changes result in different substances forming as a result of atomic rearrangement. Examples of physical and chemical changes are provided to illustrate the differences.