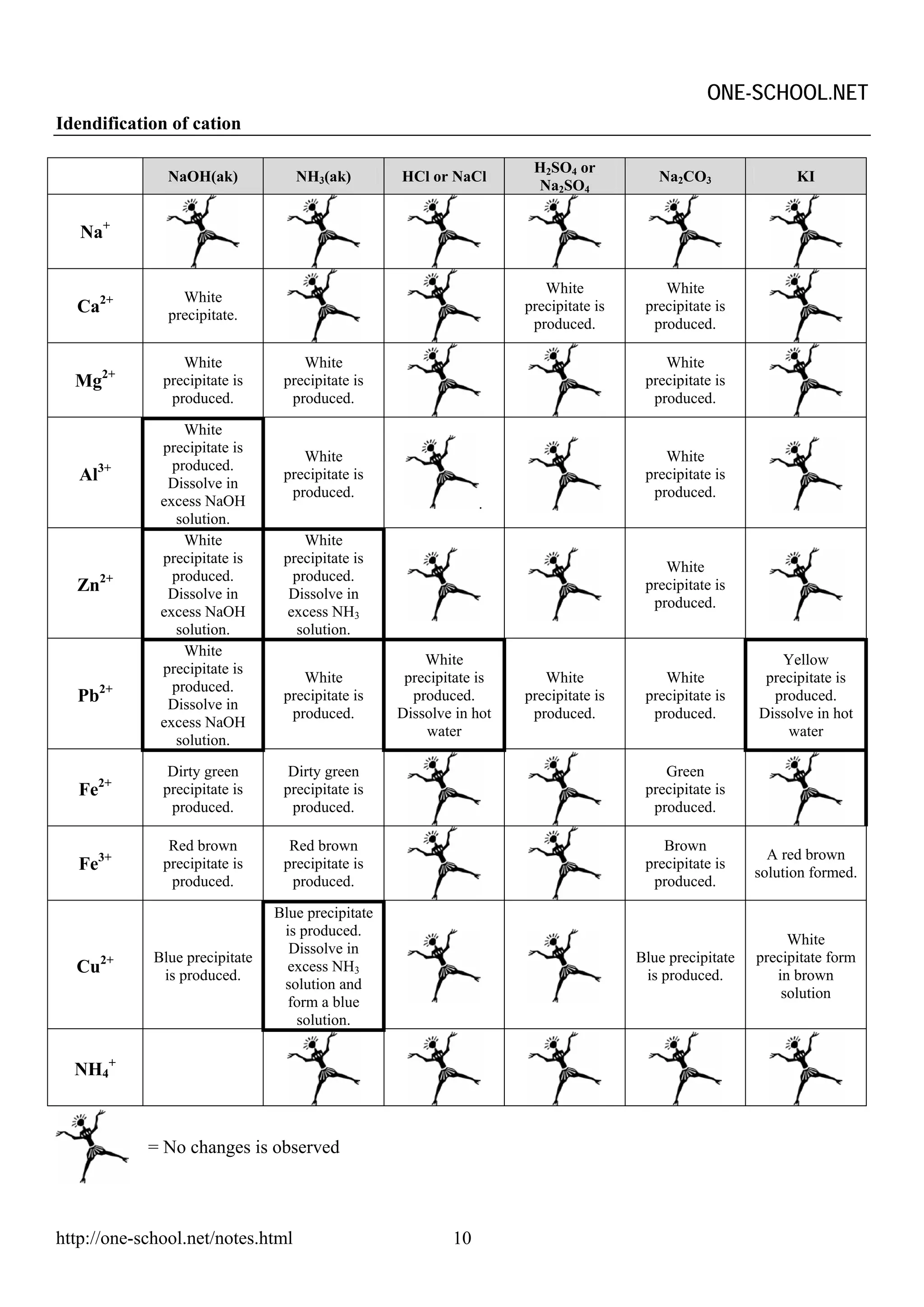
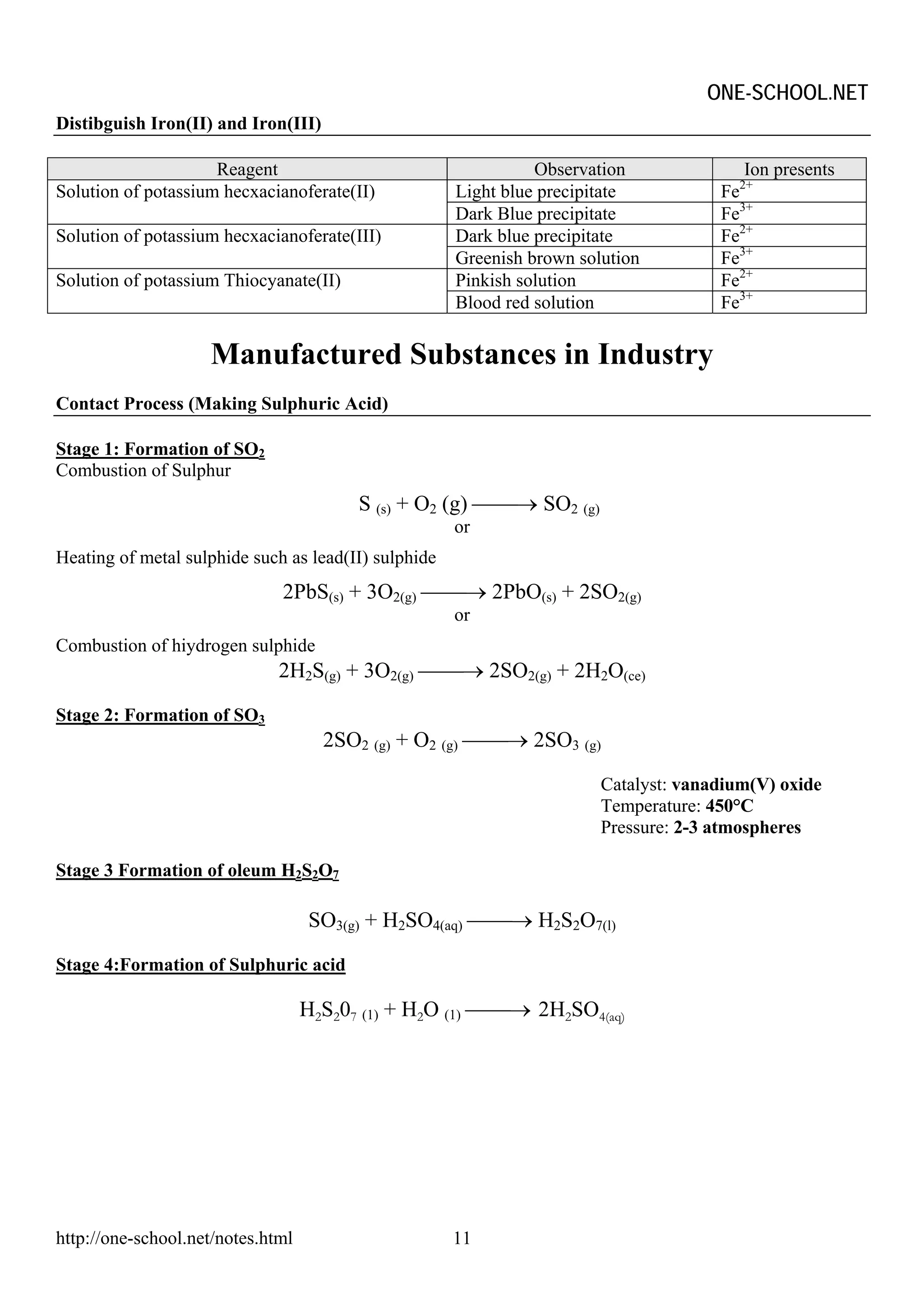
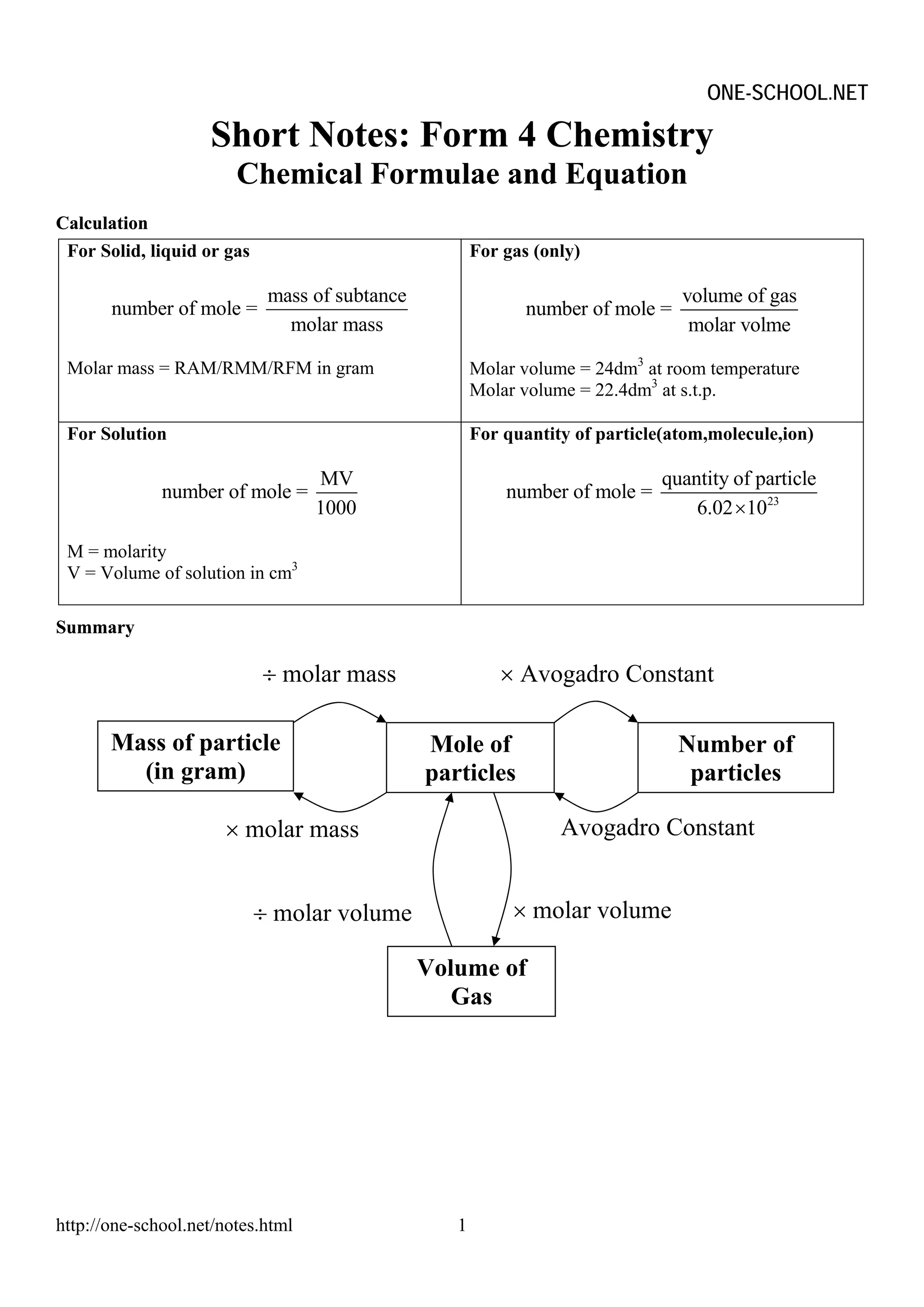
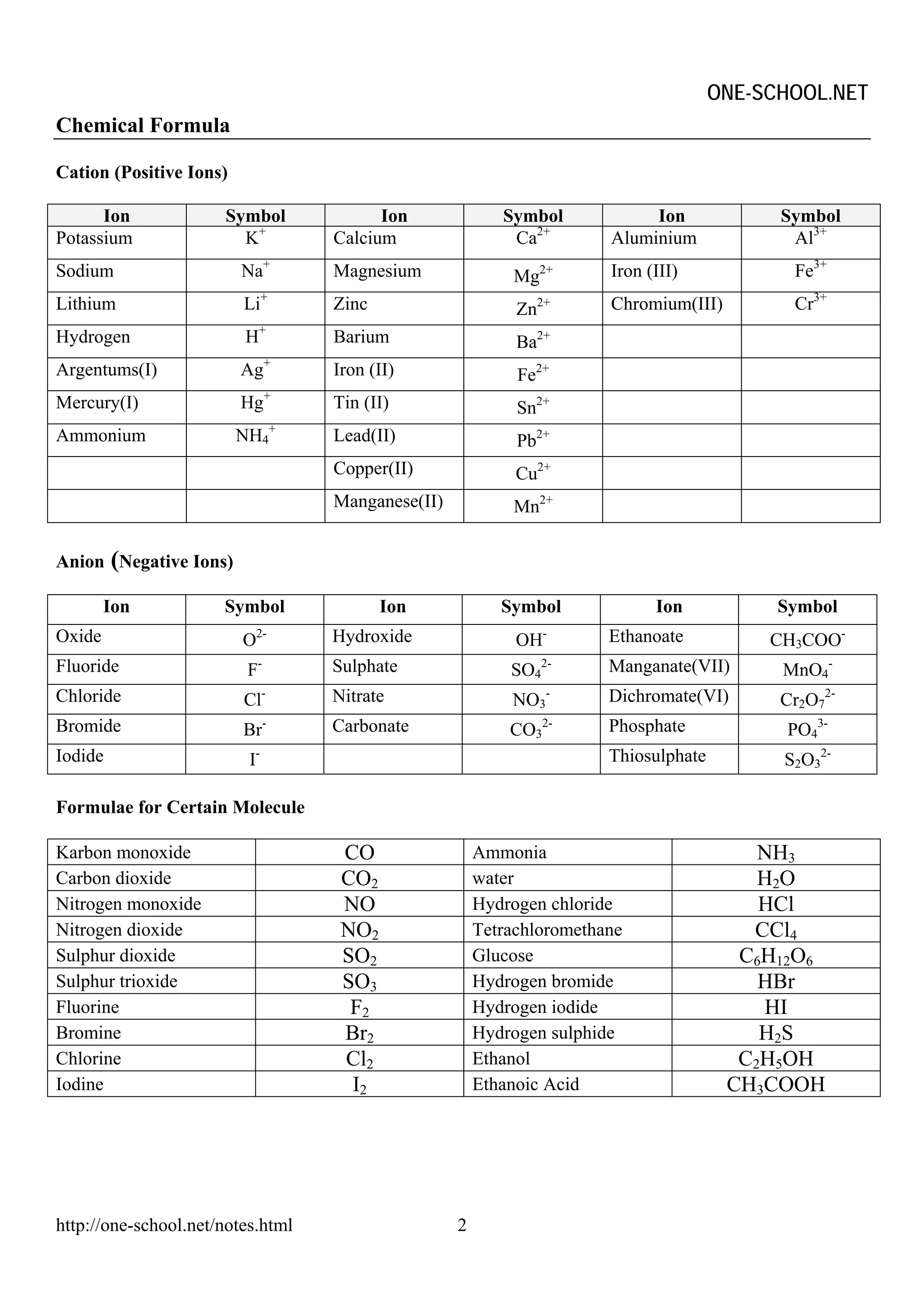
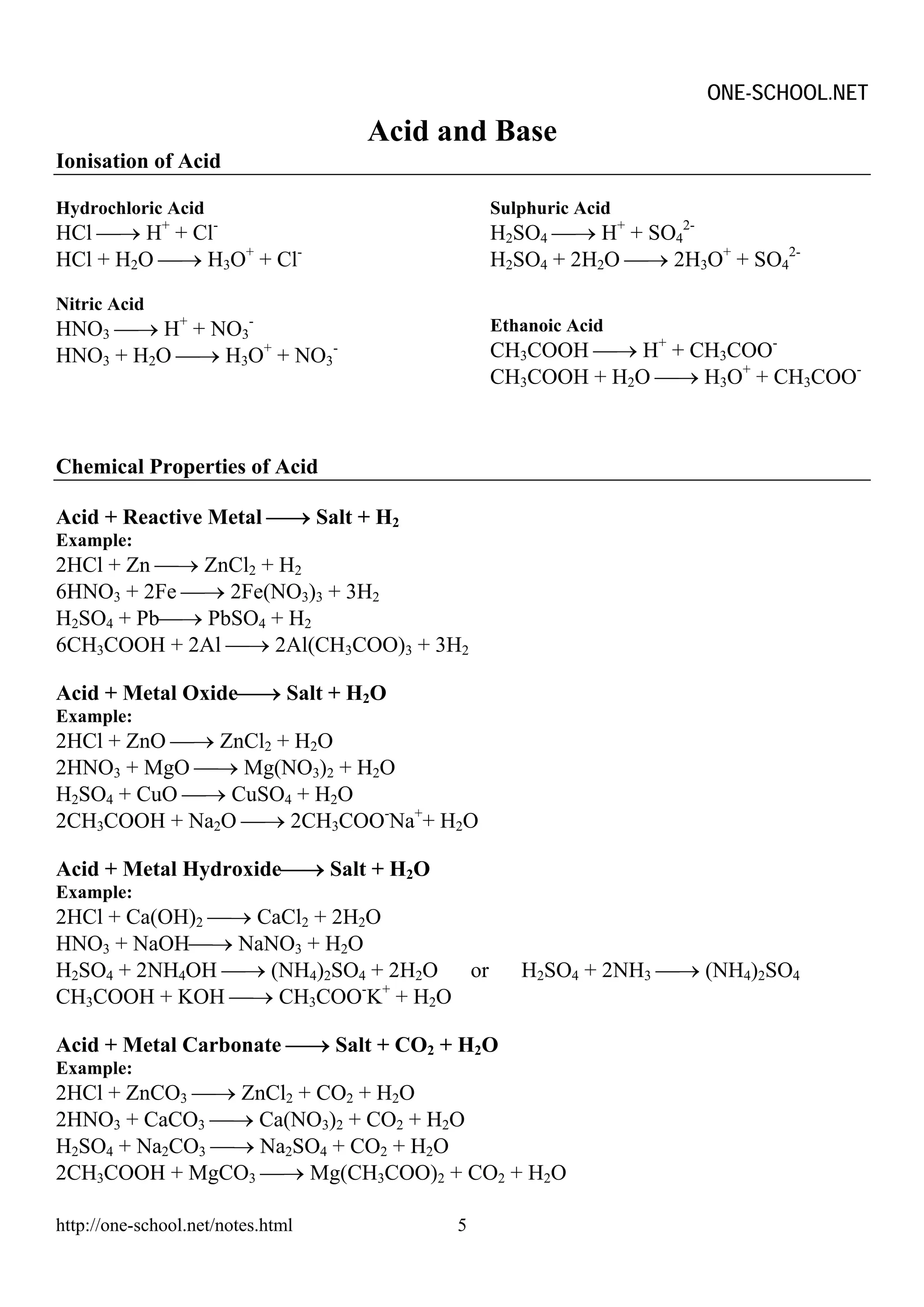
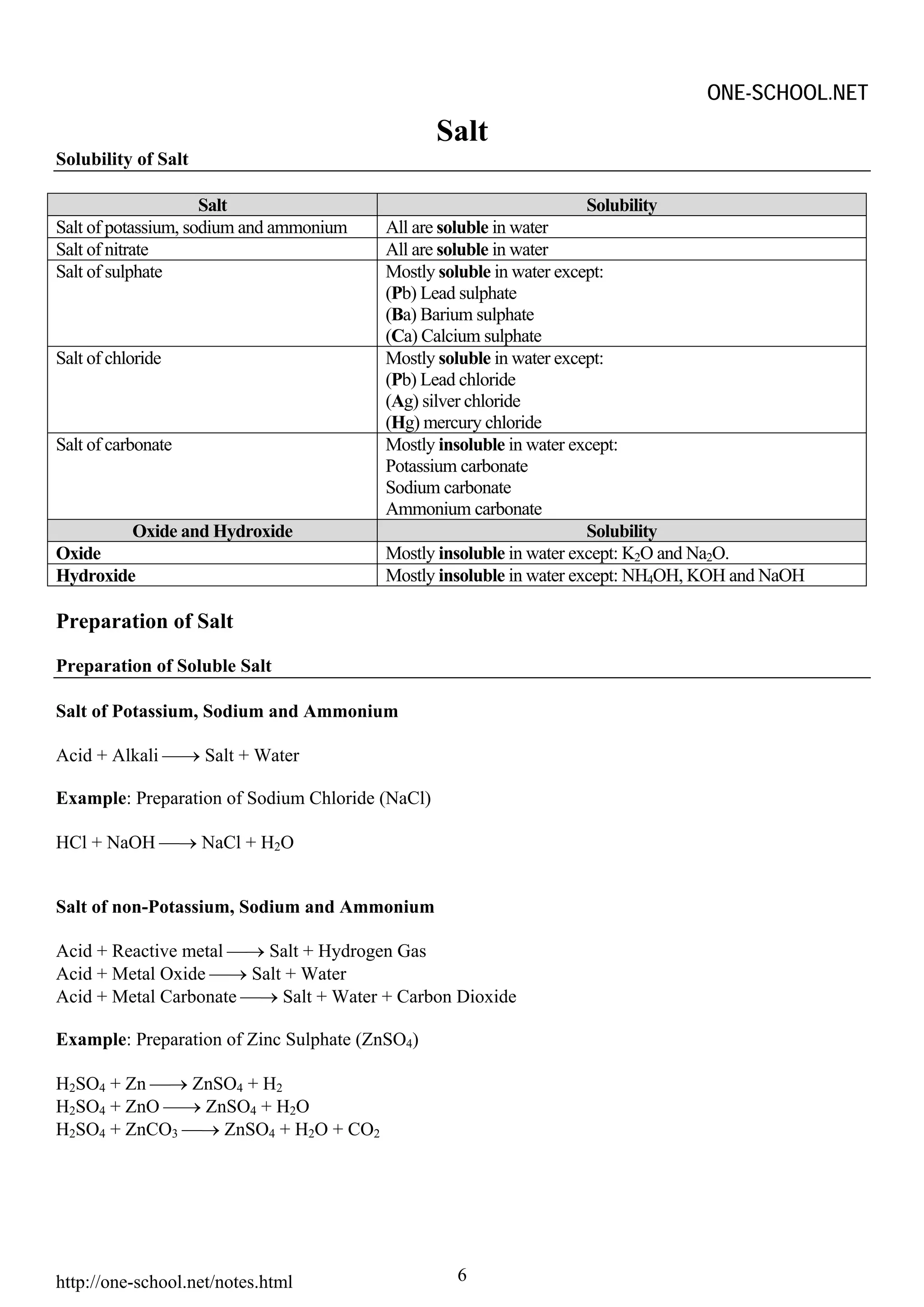
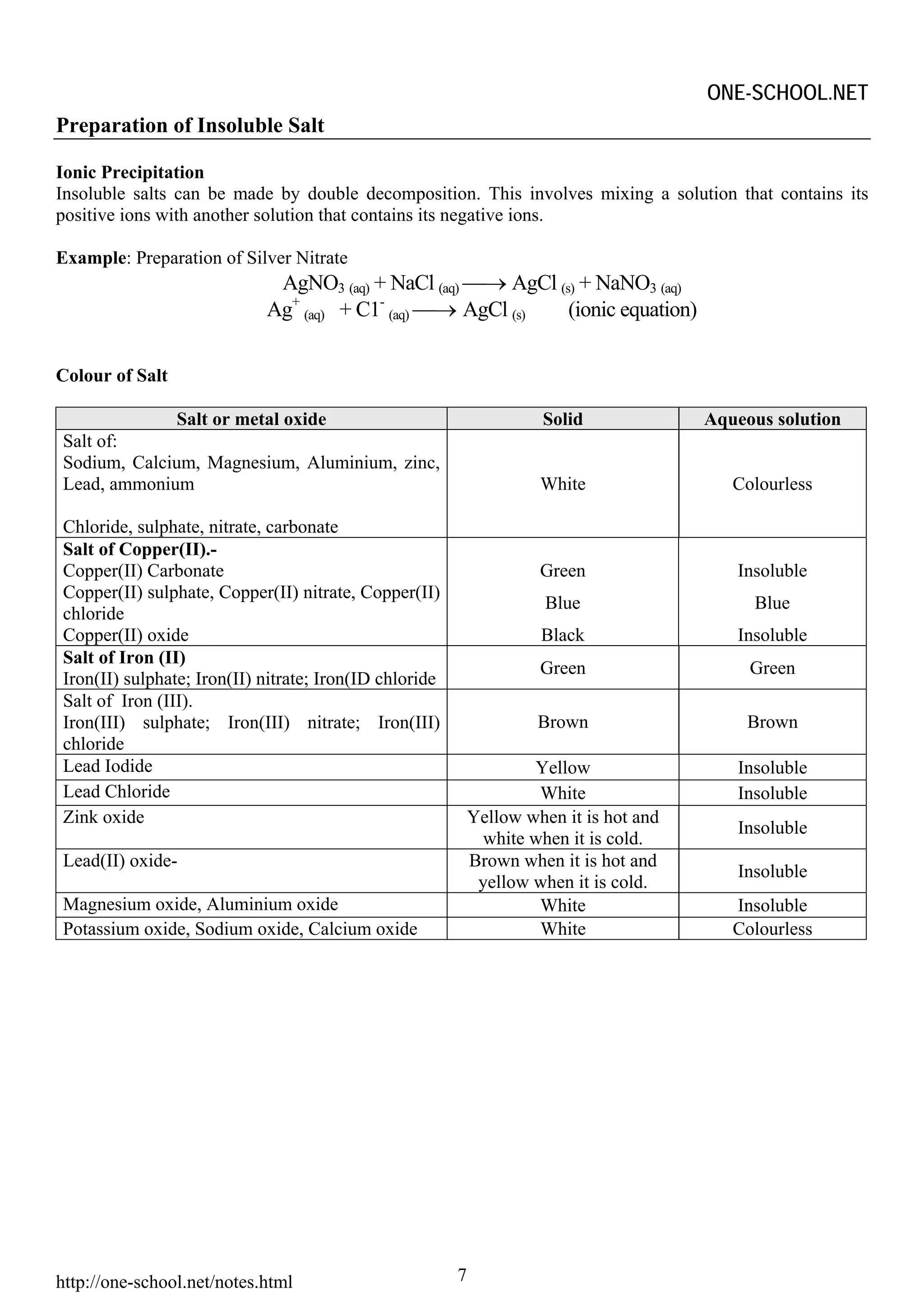
The document provides information on chemical formulae, equations, calculations involving moles and molar mass/volume. It also covers the chemical properties and reactions of group 1 and 17 elements, as well as properties of salts such as solubility, color, and the effects of heating on different salts such as carbonates and nitrates.

![ONE-SCHOOL.NET
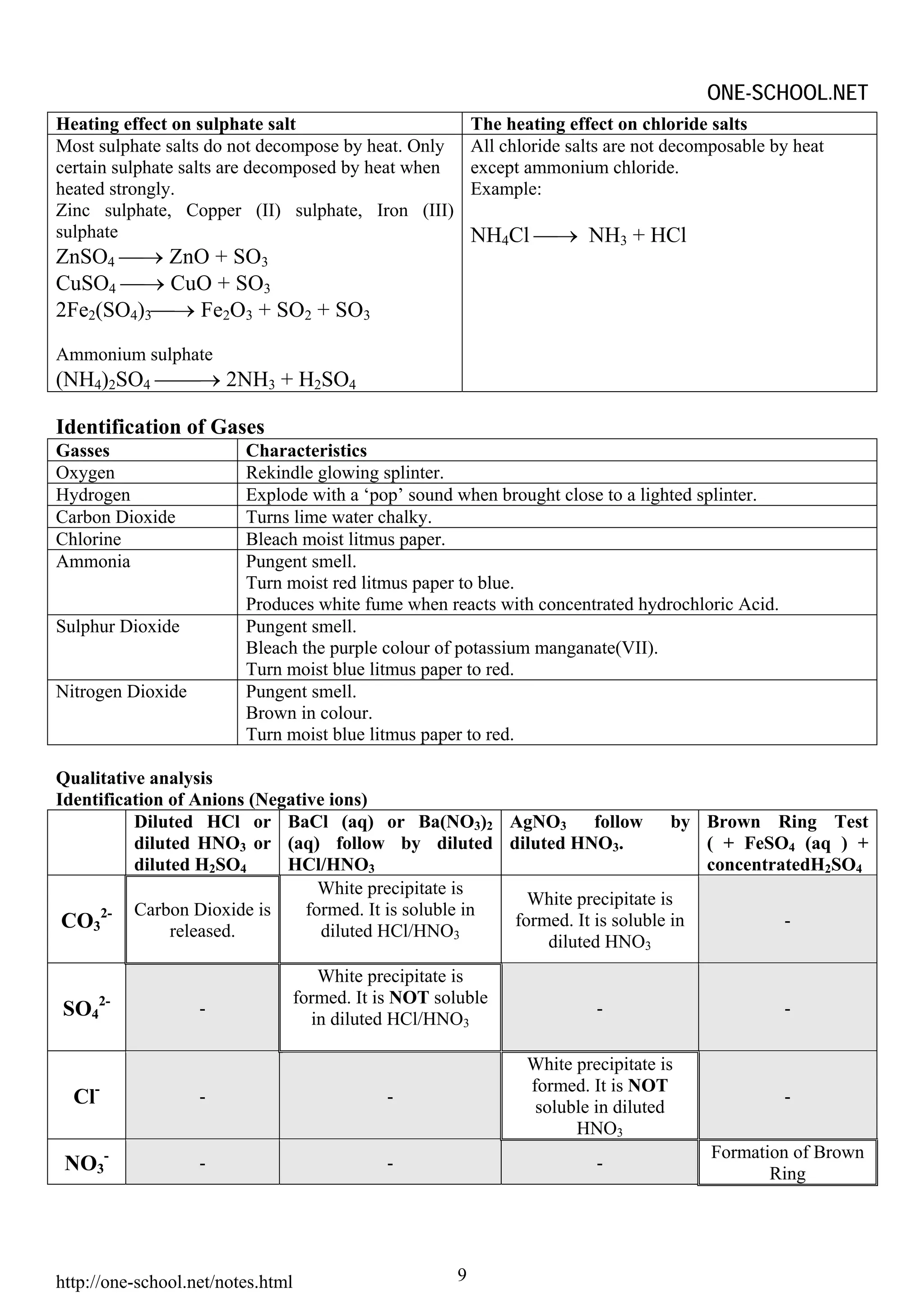
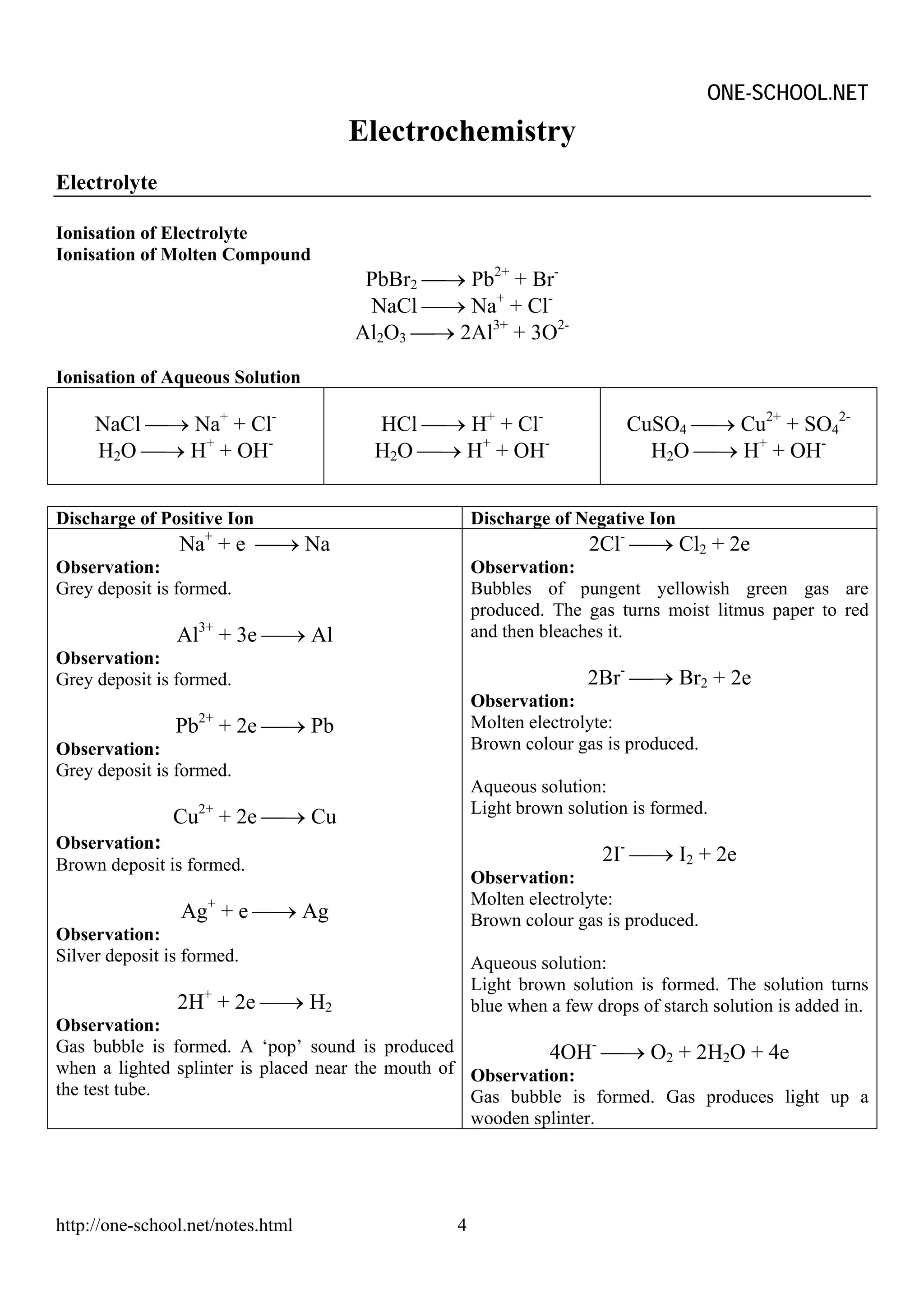
Heating effect on Salt
Heating
Effect
CO32- NO3 - SO42- Cl-
Most Probably Most Probably Most Probably Most Probably
Release CO2 Release NO2 Release SO3 No effect
Heating Effect on Carbonate Salt
Carbonate Salt Equation of The Reaction
Potassium carbonate Not decomposible
Sodium carbonate
CaCO3 ⎯→ CaO + CO2
Calcium carbonate MgCO3 ⎯→ MgO + CO2
Magnesium carbonate Al2(CO3)3 ⎯→ Al2O3 + 3CO2
Aluminium carbonate
Zinc carbonate ZnCO3 ⎯→ ZnO + CO2
Iron (III) carbonate Fe2(CO3)3⎯→ Fe2O3 + 3CO2
Lead(II) carbonate PbCO3 ⎯→ PbO + CO2
Copper(II) carbonate CuCO3 ⎯→ CuO + CO2
Mercury(II) carbonate 2HgCO3 ⎯→ 2Hg + 2CO2 + O2
Silver(I) carbonate
2Ag2CO3 ⎯→ 4Ag + 2CO2 + O2
Ammonium carbonate (NH4)2CO3 ⎯→ NH3 + CO2 + H2O
Heating Effect on Nitrate Salt
Nitrate Salt Equation of The Reaction
Potassium nitrate 2KNO3 ⎯→ 2KNO2 + O2
Sodium nitrate
2NaNO3 ⎯→ 2NaNO2 + O2
2Ca(NO3)2 ⎯→ 2CaO + 4NO2 + O2
Calcium nitrate
Magnesium nitrate Mg(NO3)2 ⎯→ 2MgO + 4NO2 + O2
Aluminium nitrate 4Al(NO3)3 ⎯→ 2Al2O3 + 12NO2 + 3O2
Zink nitrate Zn(NO3)2 ⎯→ 2ZnO + 4NO2 + O2
Iron (III) nitrate 4Fe(NO3)3⎯→ 2Fe2O3 + 12NO2 + 3O2
Lead(II) nitrate
Copper(II) nitrate Pb(NO3)2 ⎯→ 2PbO + 4NO2 + O2
Cu(NO3)2 ⎯→ 2CuO + 4NO2 + O2
Mercury(II) nitrate Hg(NO3)2 ⎯→ Hg + 2NO2 + O2
Silver(I) nitrate
2AgNO3 ⎯→ 2Ag + 2NO2 + O2
Ammonium nitrate NH4NO3 ⎯→ N2O + 2H2O
[NOTES: Nitrogen dioxide, NO2 is acidic gas and is brown in colour.]
http://one-school.net/notes.html 8](https://image.slidesharecdn.com/chemistryformulalist-1-100922132343-phpapp01/75/Chemistry-formula-list-1-Examville-com-8-2048.jpg)