Embed presentation
Downloaded 27 times
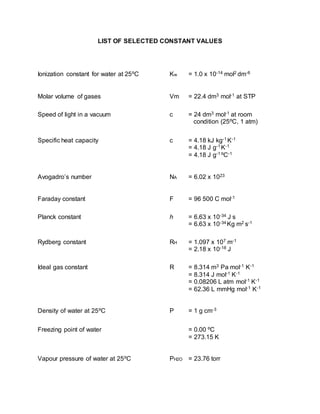


This document lists several important physical constants and conversion factors used in chemistry. It includes the ionization constant of water, molar volume of gases, speed of light, specific heat capacity, Avogadro's number, Faraday's constant, Planck's constant, Rydberg constant, ideal gas constant, density of water, freezing point of water, and vapor pressure of water. Conversion factors provided relate units of volume, energy, pressure, and temperature.

