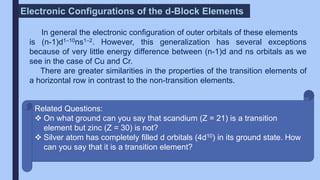
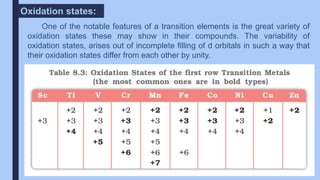
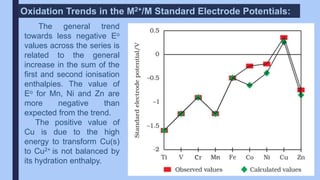
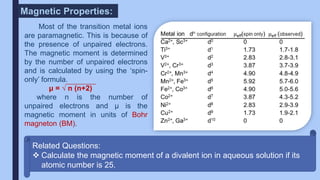
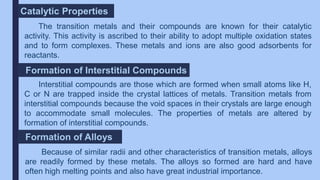
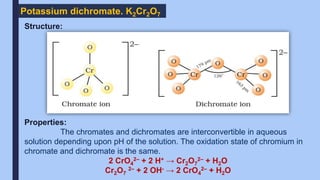
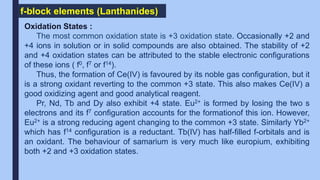
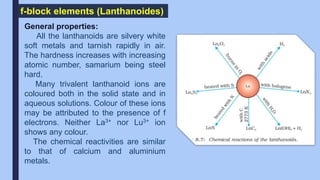
The document discusses the d-block and f-block elements of the periodic table. It provides details about their electronic configurations, properties, and reactions. The d-block contains the transition metals whose d orbitals are filled from groups 3 to 12. The f-block contains the inner transition metals whose 4f and 5f orbitals are filled. Elements in these blocks can exhibit a variety of oxidation states and form complexes due to their electronic structure.









![f-block elements (Lanthanoides)
Introduction:
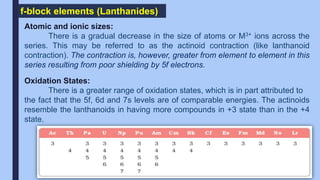
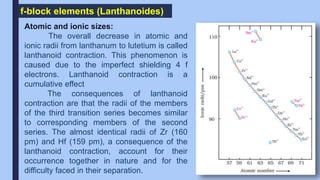
The f-block elements are called inner transition elements. The outermost
electron is filled in the f-orbital of the antepenultimate shell. The general
electronic configuration of lanthanides is [Xe] 4f1-14 5d0,1 6s2.](https://image.slidesharecdn.com/dandfblock-211125134842/85/D-and-f-block-22-320.jpg)

![f-block elements (Actinoides)
Electronic configuration:
The general electronic configuration of actinoides is [Rn] 5f1-14 6d0,1 7s2.
The elements after Uranium are called trans-uranic elements and are artificial
and radioactive.](https://image.slidesharecdn.com/dandfblock-211125134842/85/D-and-f-block-27-320.jpg)