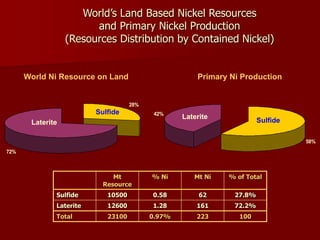
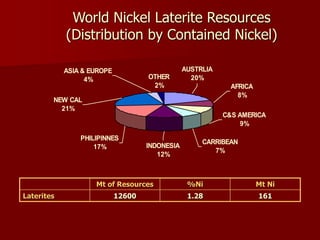
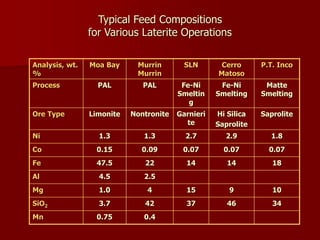
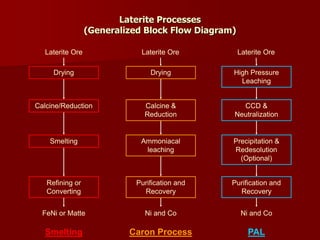
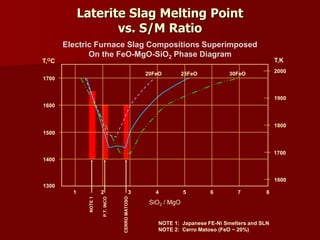
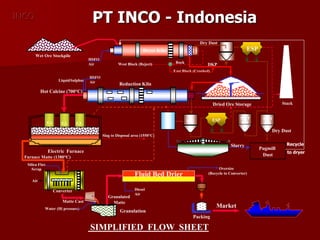
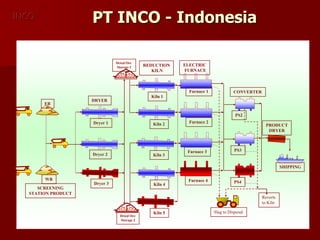
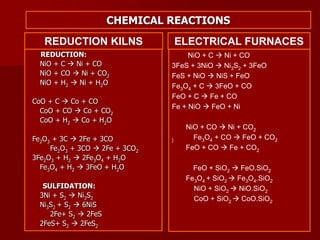
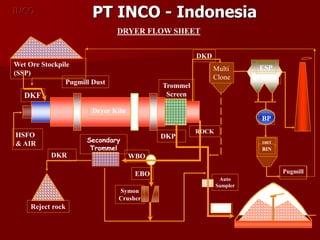
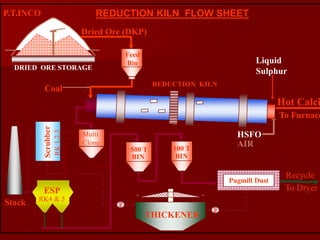
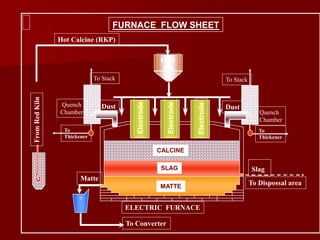
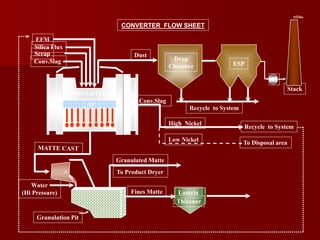
The document provides information on nickel/cobalt laterite processes including mineralogy, typical ore compositions, process routes, and examples of operating plants. It describes the key steps in pyrometallurgical processes including ore preparation, drying, calcination/reduction, smelting to produce ferronickel or matte, and refining. The pressure acid leach and reduction roast-ammonia leach hydrometallurgical processes are also briefly covered. Process flowsheets for INCO's nickel operations in Indonesia illustrate ore drying, reduction kilning, electric furnace smelting, and matte converting steps.