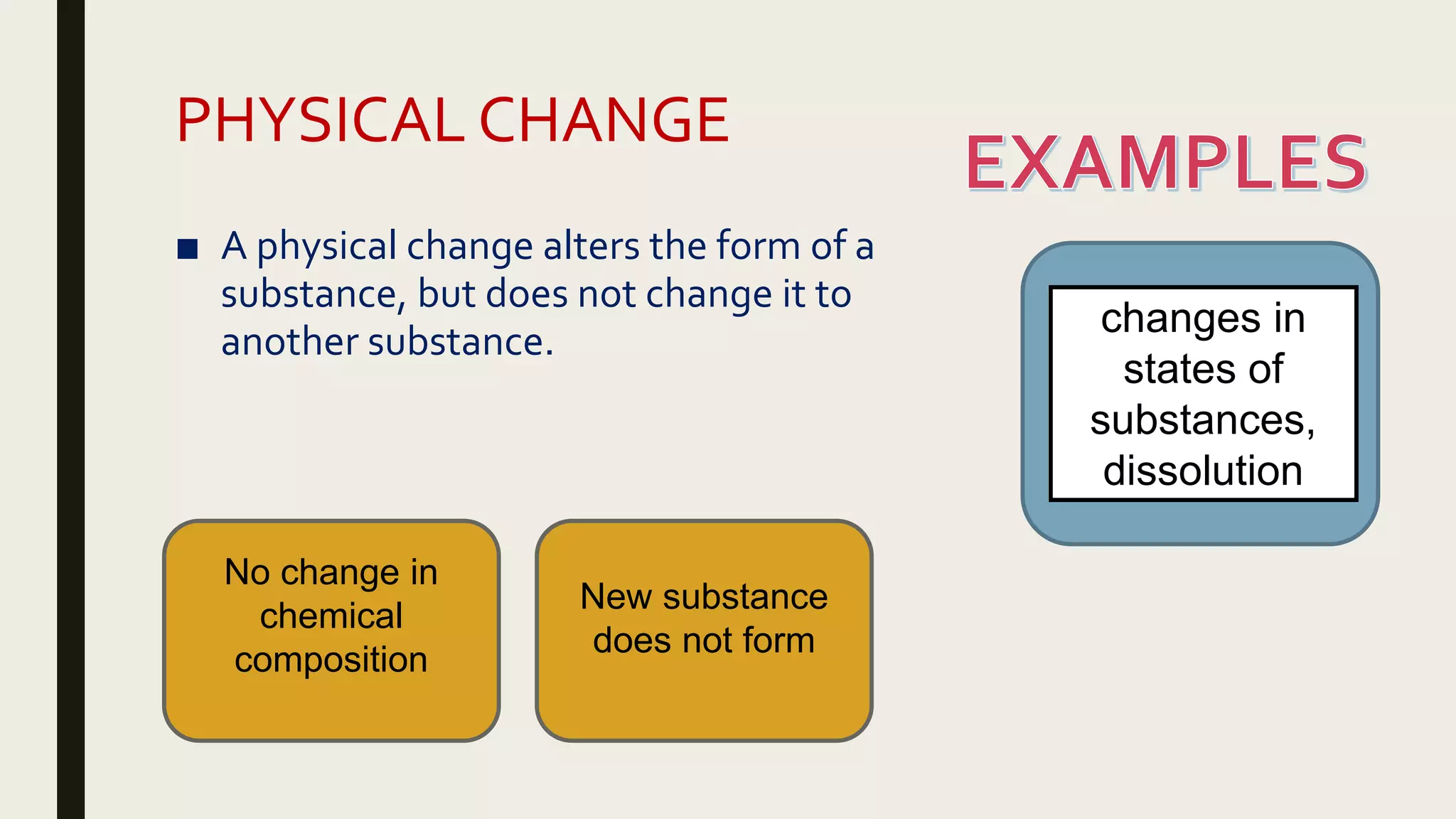
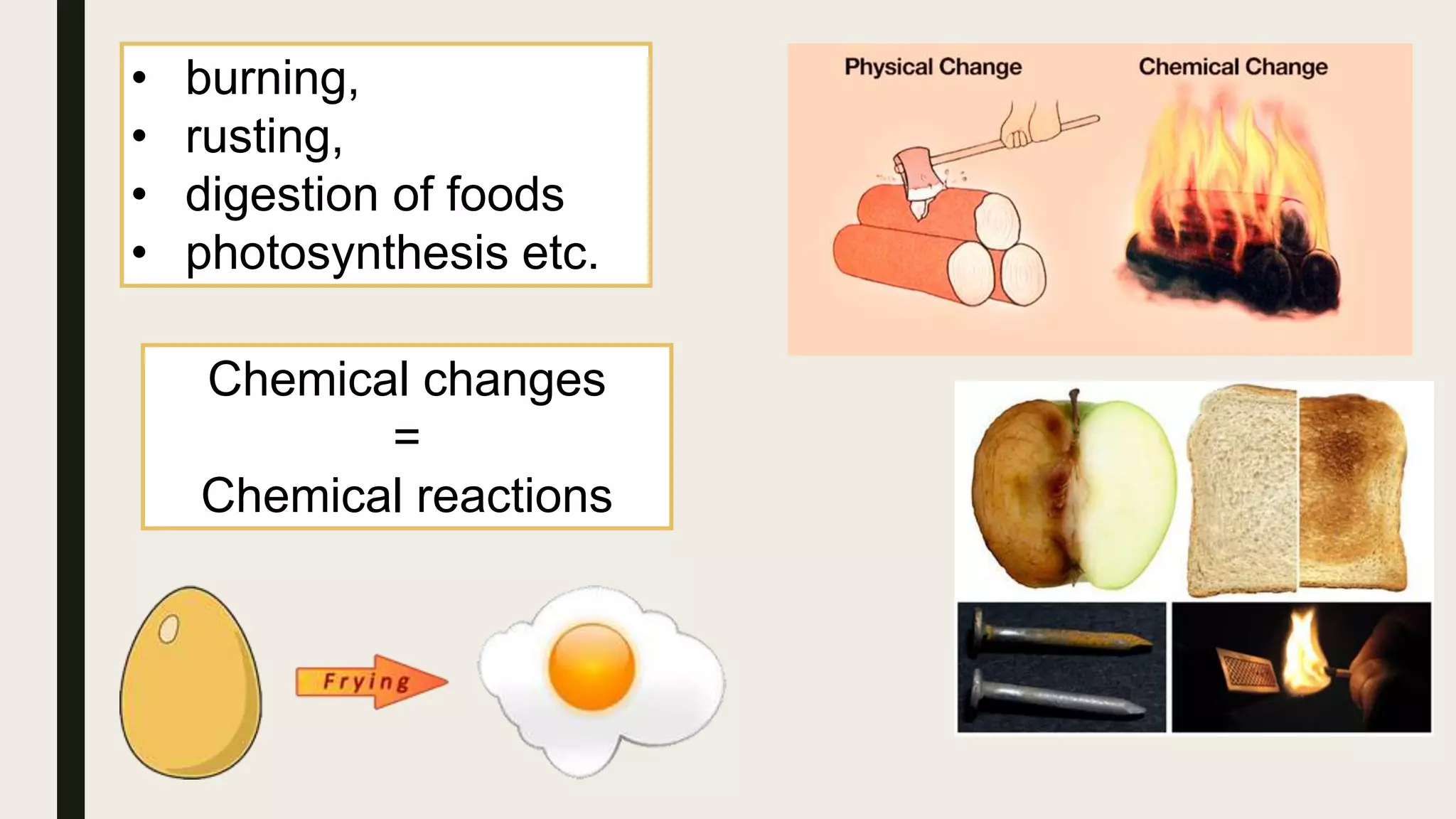
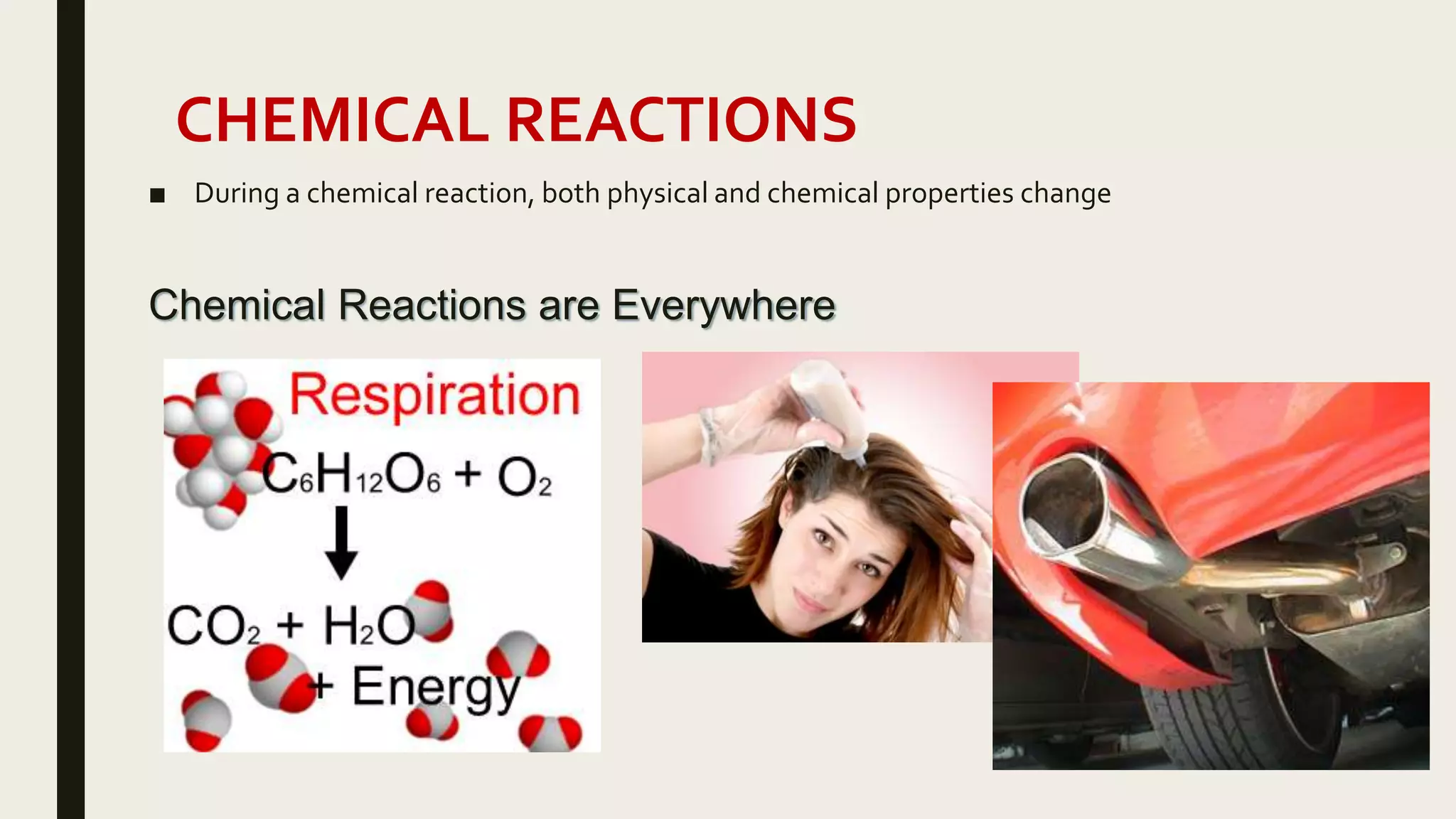
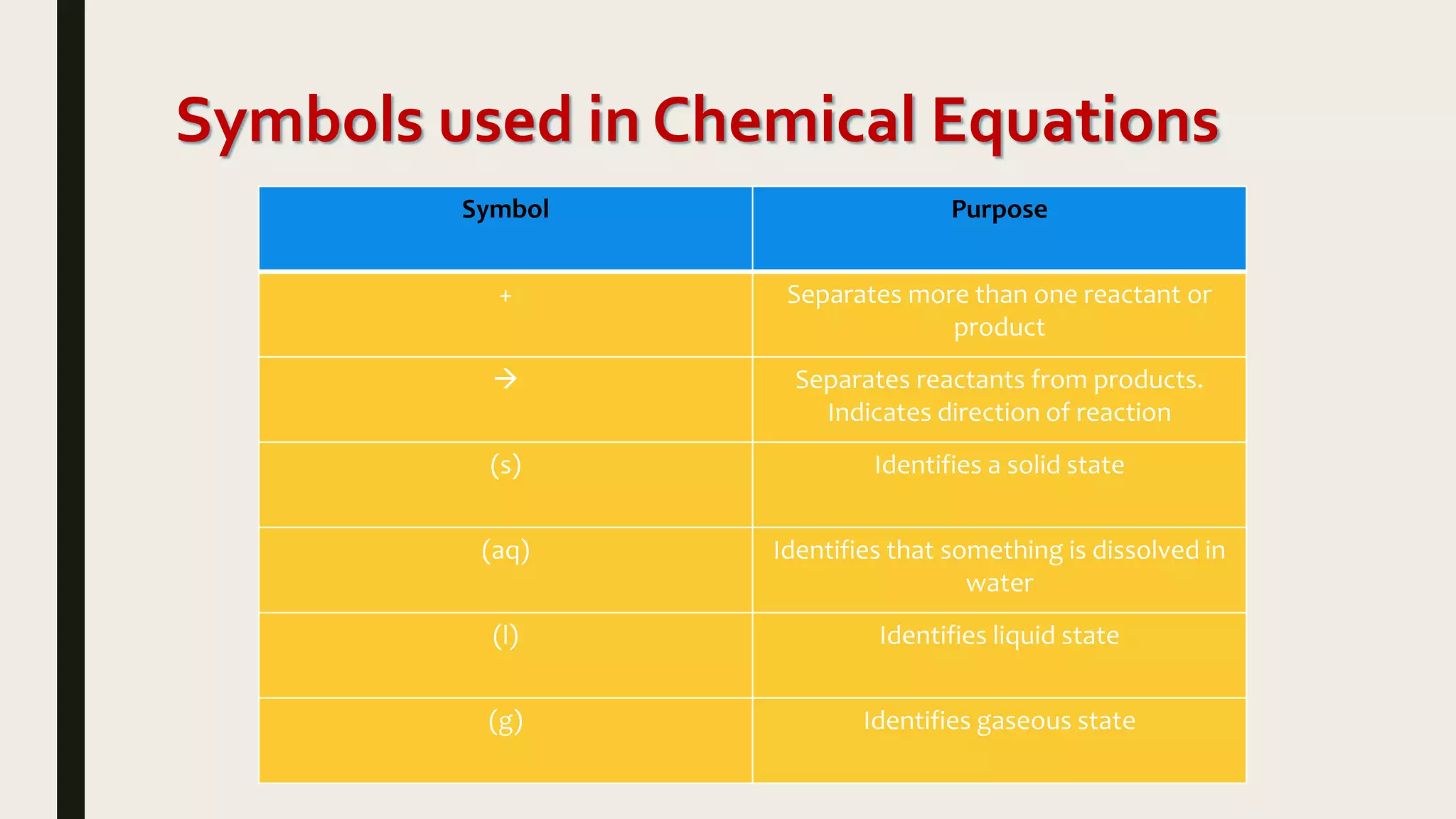
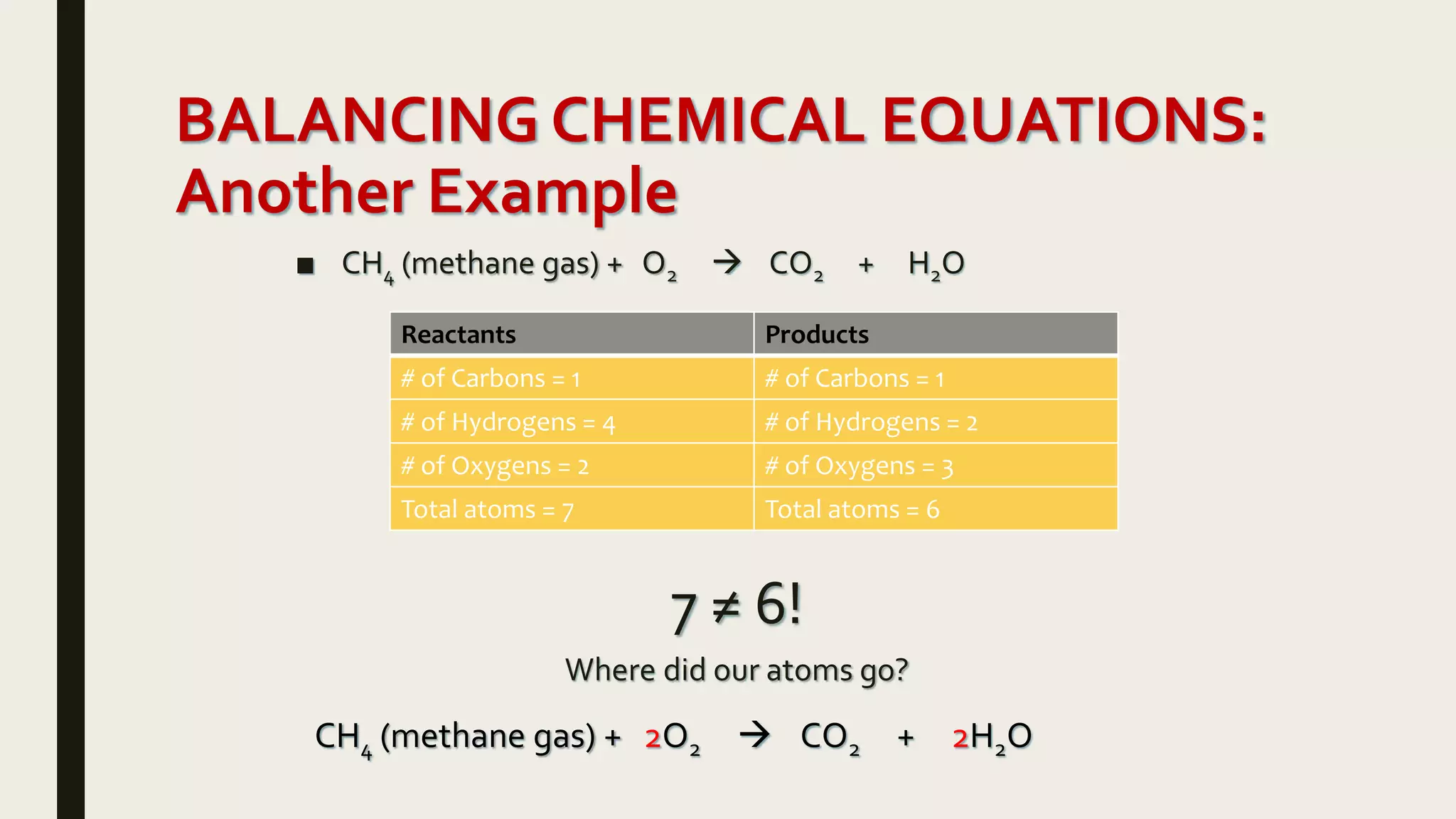
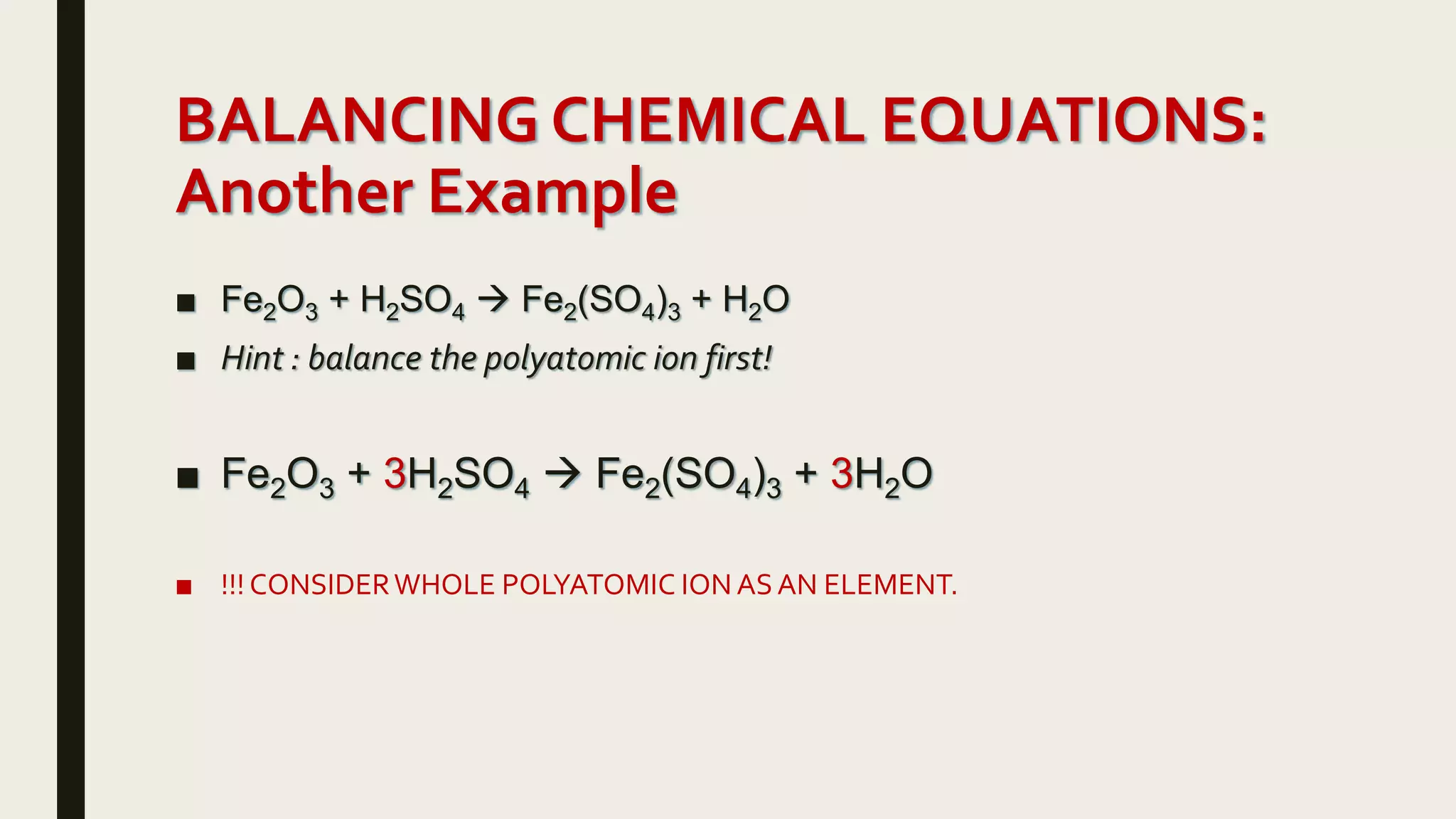
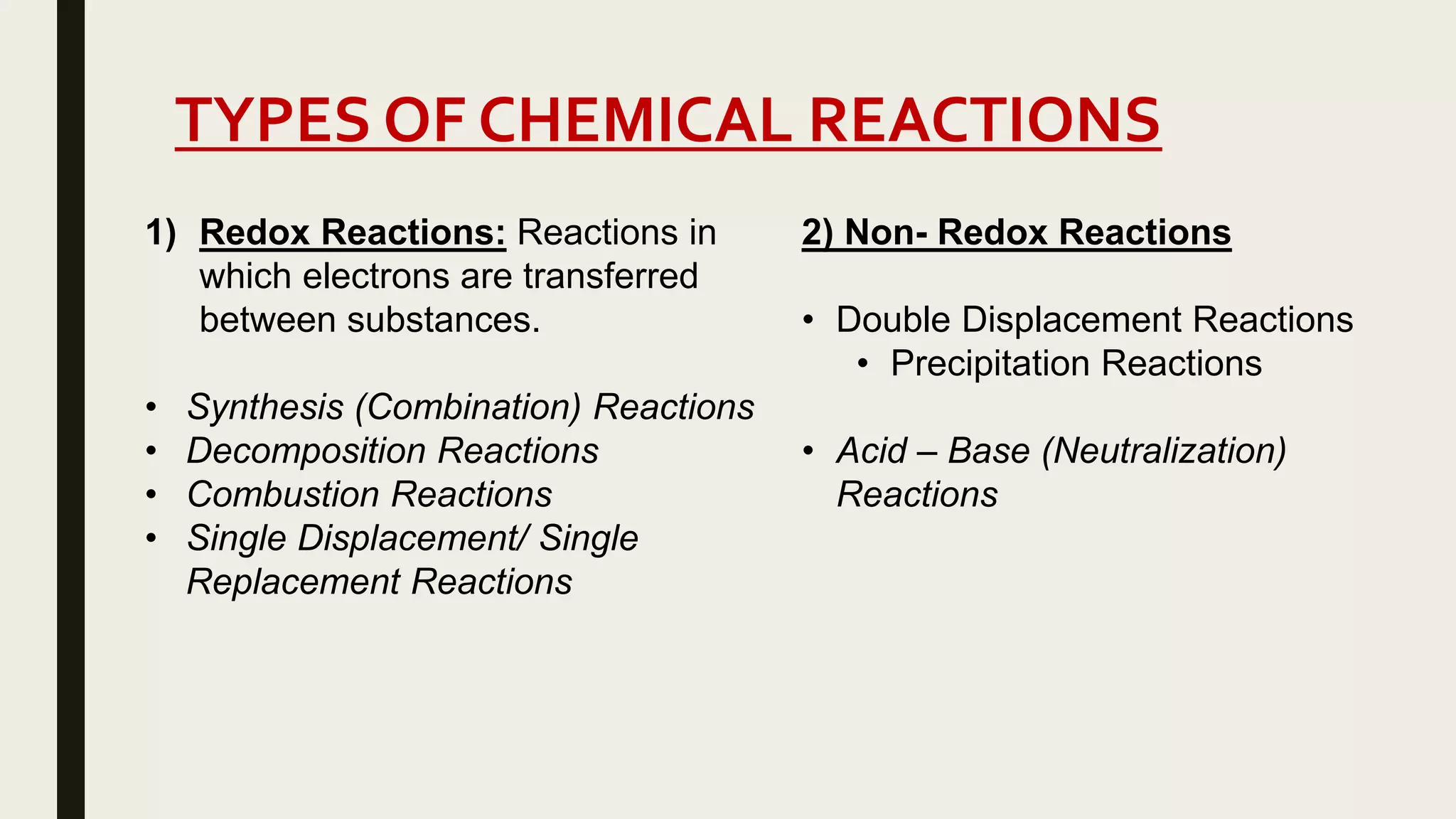
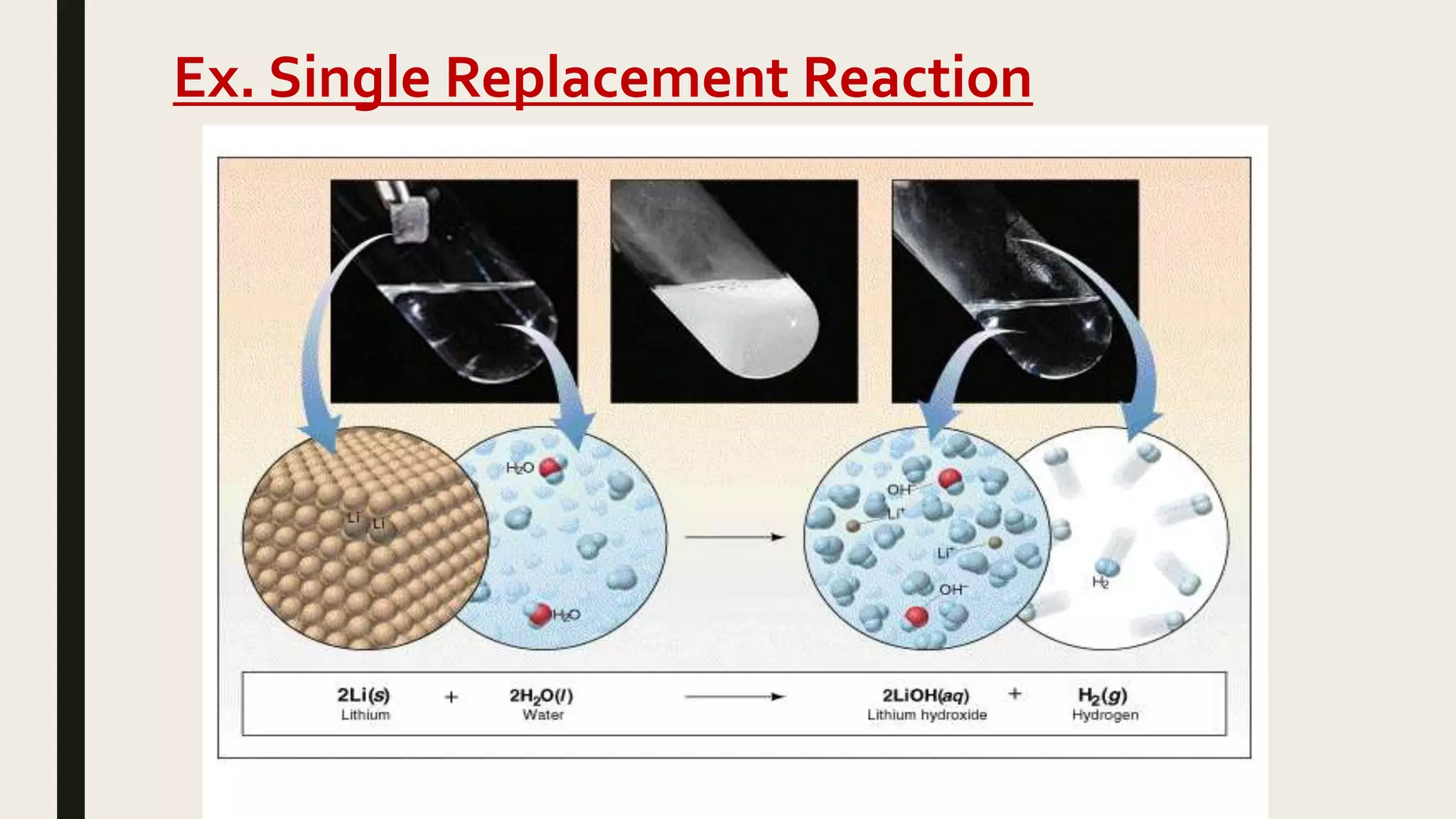
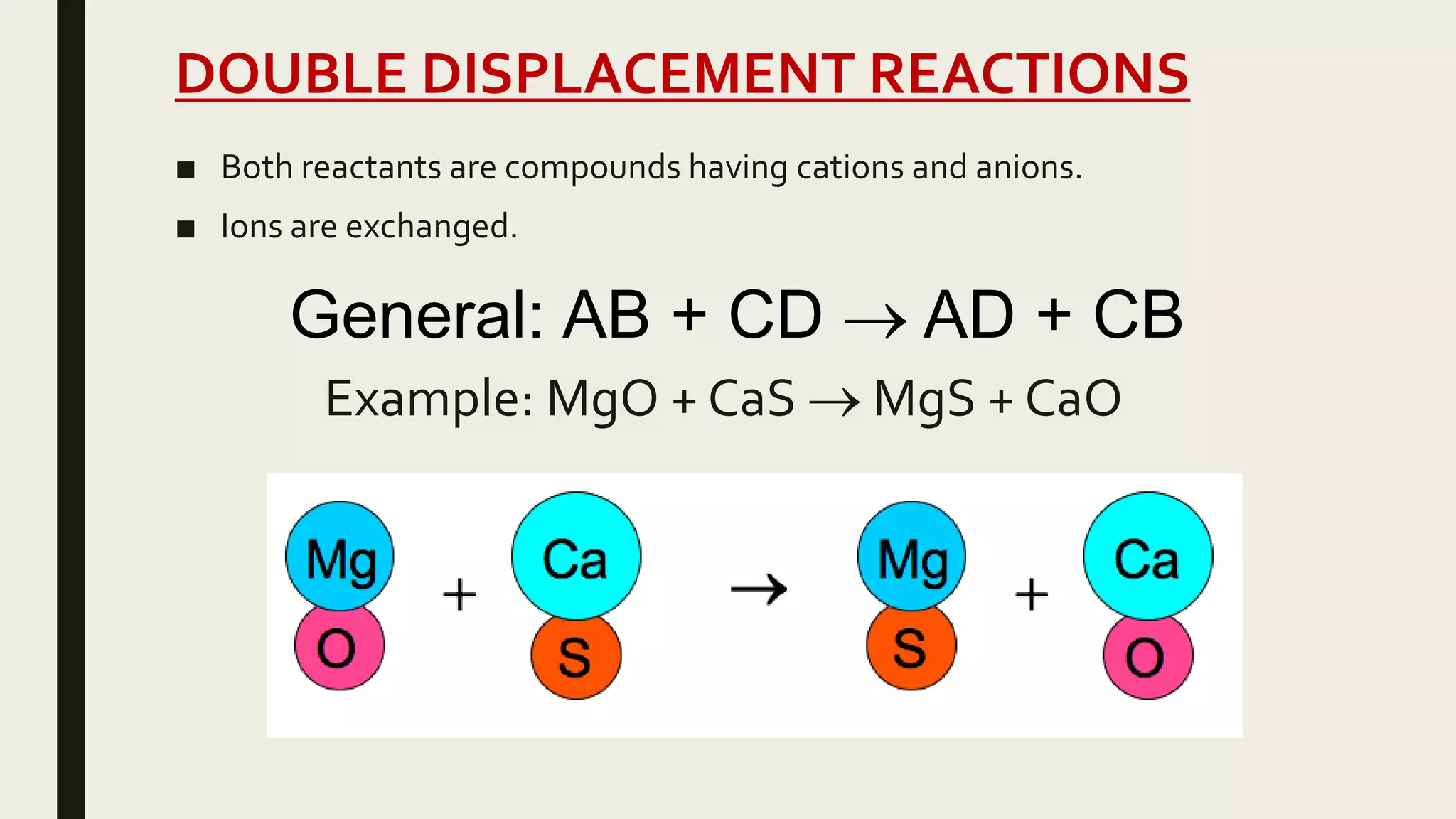
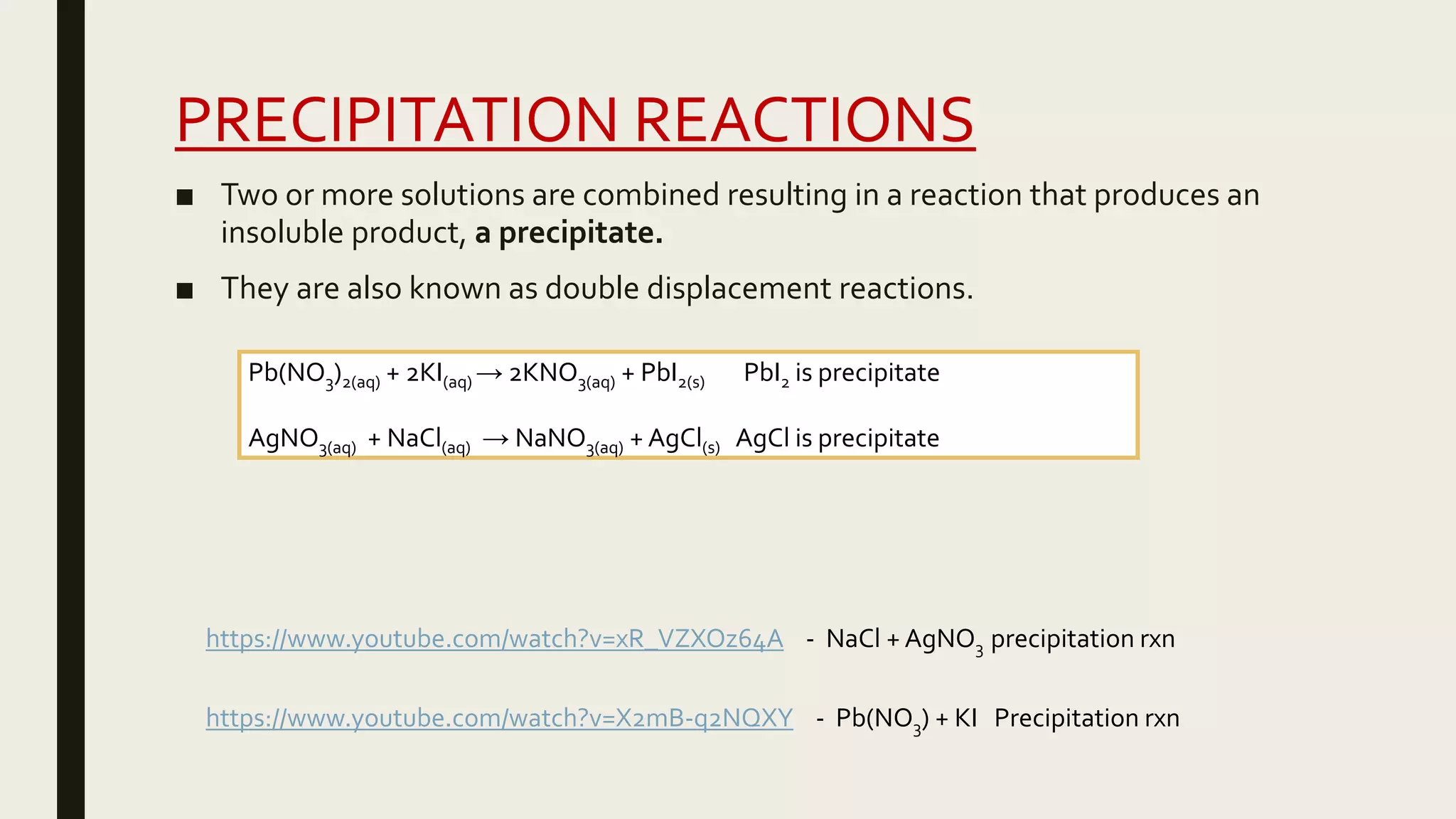
This document summarizes different types of chemical and physical changes. It describes physical changes as changes in a substance's form or state without altering its chemical composition. Chemical changes form new substances through rearrangement of atoms. The document outlines various chemical reactions including synthesis, decomposition, combustion, single and double displacement, precipitation, and acid-base reactions. It emphasizes that chemical equations represent chemical reactions and must balance the number and type of atoms on each side.